Comprehensive Guide to Battery Classification: A Complete Reference
- a. Lithium-ion batteries
-
The previous article has actually mentioned the lithium-ion battery many times. I believe you already understand its basic concept. (Related airticle: The Ultimate Guide To Batteries) But many people often confuse many concepts, such as lithium-ion batteries, lithium iron phosphate batteries and so on. Here it comes to the lithium-ion battery classification. Please continue reading below.
Lithium-ion batteries can be classified into several categories based on their construction and composition. Here are some common classifications of lithium-ion batteries:
1. Lithium Cobalt Oxide (LiCoO2) batteries: These are one of the most widely used types of lithium-ion batteries, commonly found in consumer electronics such as smartphones and laptops.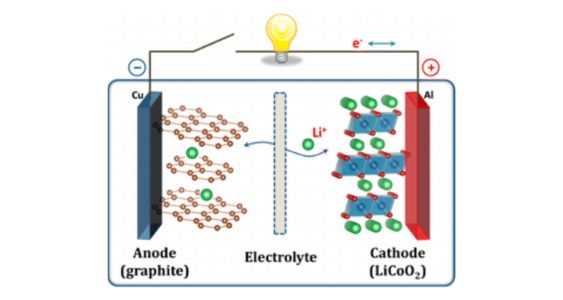
Main components: a cathode (positive electrode) made of lithium cobalt oxide, an anode (negative electrode) typically made of graphite, and a separator that allows the flow of lithium ions between the electrodes while preventing direct contact.
• Energy density: Approximately 150-200 Wh/kg
• Cycle life: Around 300-500 cycles
• Self-discharge rate: About 5-8% per month
2. Lithium Iron Phosphate (LiFePO4) batteries: These batteries are known for their excellent safety performance and long cycle life. They are often used in electric vehicles (EVs) and energy storage systems.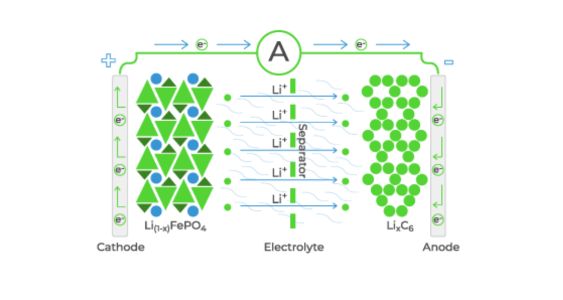
Main components: LiFePO4 batteries consist of a cathode (positive electrode) made of lithium iron phosphate, an anode (negative electrode) typically made of carbon, and a separator that allows the flow of lithium ions while preventing direct contact between the electrodes.
• Energy density: Around 130-160 Wh/kg
• Cycle life: Typically 2000-5000 cycles
• Self-discharge rate: Approximately 1-3% per month
3. Lithium Nickel Manganese Cobalt Oxide (LiNiMnCoO2 or NMC) batteries: NMC batteries offer a balance between energy density, power capability, and safety. They are commonly used in electric vehicles and portable electronic devices.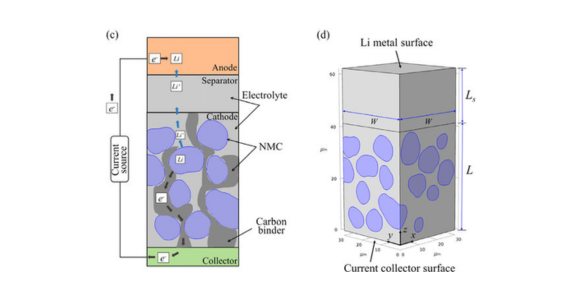
Main components: The composition of NMC batteries can vary, but the most common formulation is a ratio of nickel, manganese, and cobalt in the cathode, such as NMC 111 (equal parts nickel, manganese, and cobalt) or NMC 532 (5 parts nickel, 3 parts manganese, and 2 parts cobalt). The exact ratio affects the performance characteristics of the battery, including energy density, power density, and cycle life.
• Energy density: Approximately 200-250 Wh/kg
• Cycle life: Typically 500-1000 cycles
• Self-discharge rate: About 3-5% per month
4. Lithium Nickel Cobalt Aluminum Oxide (LiNiCoAlO2 or NCA) batteries: NCA batteries are known for their high energy density and are used in electric vehicles, such as some models produced by Tesla.
Main components: The composition of NCA batteries typically consists of a high concentration of nickel, a moderate amount of cobalt, and a small amount of aluminum in the cathode material. This formulation allows for a high energy density and good overall performance.
• Energy density: Around 200-260 Wh/kg
• Cycle life: Approximately 500-1000 cycles
• Self-discharge rate: Approximately 2-3% per month
5. Lithium Titanate (Li4Ti5O12) batteries: These batteries have a high rate capability and long cycle life, making them suitable for applications that require fast charging and high power output, such as electric buses and grid energy storage.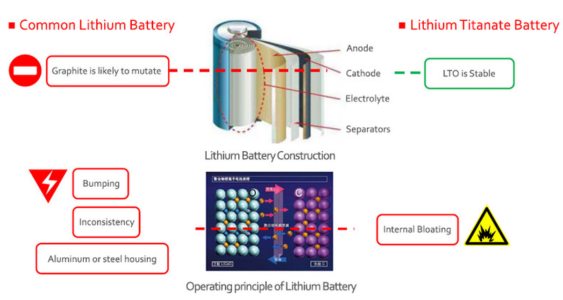
Main components: The cathode material in Li4Ti5O12 batteries is composed of lithium titanium oxide, which has a spinel crystal structure. This structure allows for the insertion and extraction of lithium ions with minimal strain, enabling the battery to achieve a long cycle life.
• Energy density: Typically 80-120 Wh/kg
• Cycle life: Around 10,000 cycles or more
• Self-discharge rate: About 1-2% per month
6. Lithium-Sulfur (Li-S) batteries: Li-S batteries have the potential to offer high energy density, but they are still under development and not widely commercialized.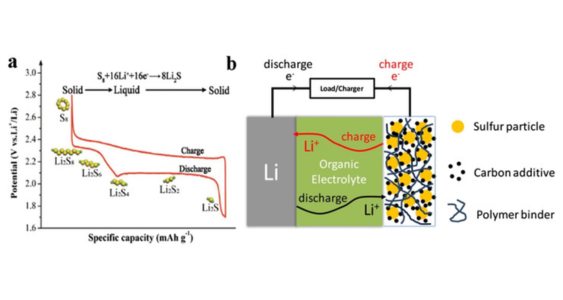
Main components: The cathode of Li-S batteries is typically composed of elemental sulfur or sulfur compounds, while the anode can be lithium metal or a lithium-ion host material. During discharge, lithium ions shuttle between the anode and cathode through the electrolyte, and sulfur undergoes a series of chemical reactions to form lithium sulfide compounds. The reverse process occurs during charging.
• Energy density: Currently under development, but potentially over 300 Wh/kg
• Cycle life: Still being improved, typically around 200-500 cycles
• Self-discharge rate: Varies depending on the specific design and chemistry
7. Solid-State Lithium-ion batteries: These batteries use a solid electrolyte instead of a liquid or gel electrolyte, offering potential advantages in terms of safety, energy density, and cycle life. However, they are still in the research and development stage.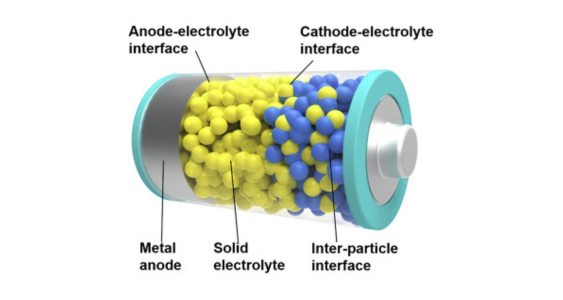
Main components: In solid-state lithium-ion batteries, both the cathode and anode are typically made of lithium-containing materials, similar to traditional lithium-ion batteries. However, the key difference lies in the electrolyte, which is a solid material that facilitates the transport of lithium ions between the electrodes.
• Energy density: Currently under development, but potentially exceeding 500 Wh/kg
• Cycle life: Still being researched, but expected to be significantly higher than conventional lithium-ion batteries
• Self-discharge rate: Expected to be lower than conventional lithium-ion batteries, but specific data is not yet widely available.
These are just some of the common types, and there are other specialized types of lithium-ion batteries under development.
- b. Lithium Iron Phosphate Battery
-
The previous article has actually mentioned the concept of lithium iron phosphate batteries, which is a member of the lithium-ion battery family. But because of its special properties, I have to talk about it in more detail separately.
Lithium-iron phosphate batteries have the following unique features compared to traditional lithium-ion batteries: high safety, long cycle life, lower risk of thermal runaway and a wider operating temperature range. Lithium-iron phosphate batteries use lithium ions between the positive and negative electrodes as the cathode material, which has more stable chemical properties and can provide higher safety and longer cycle life. In addition, lithium-iron phosphate batteries have a lower risk of thermal runaway compared to conventional lithium-ion batteries under extreme conditions such as high temperature or overcharging. This makes lithium-iron phosphate batteries more advantageous in some applications that require higher safety and can operate properly over a wider temperature range.
The following are common parameters for lithium-iron phosphate batteries:
Temperature Range: Lithium-iron phosphate batteries typically operate over a wide temperature range, typically from -20 degrees Celsius to 60 degrees Celsius.
Self-discharge Rate: The self-discharge rate is the rate at which a battery loses power on its own when not in use. The self-discharge rate of LiFePO4 battery is 1-3% per month.
Cycle Efficiency: Cycle efficiency refers to the percentage of energy lost during the charge/discharge cycle of the battery. Lithium-iron phosphate batteries usually have a high cycle efficiency and are able to convert electrical energy into chemical energy and release it with high efficiency.
Battery Size: Lithium-iron phosphate batteries are available in the market in a variety of different sizes and shapes, such as 18650, 26650, etc.
Battery shape: Prismatic or Cylindrical.
Nominal Voltage: The nominal voltage of a single lithium-iron phosphate battery is 3.2 volts (V).
Cut-off Voltage: The cut-off voltage of a single lithium-iron phosphate battery is generally 2.5 volts
Capacity: The capacity of cylindrical LiFePO4 cells typically ranges from 1000 mAh to 3000 mAh or higher. Square LiFePO4 cells have a broader capacity range from 7Ah to 400Ah or higher.
Charging Rate: The charging rate is usually expressed as a C value, which is a multiple of the battery capacity. For example, a charging rate of 1C means that the battery is charged at the same current as its capacity. A typical LiFePO4 battery can support charging rates as high as 1C to 2C or even higher.
Discharge Rate: The discharge rate, also expressed as a C value, represents the ratio of the battery's continuous discharge current to its capacity. Lithium-iron phosphate batteries usually have a high discharge rate capability and can support discharge rates of up to 3C or higher.
Life (Cycle Life): Lithium-iron phosphate batteries usually have a long life, can withstand 2000-5000 cycles of charge and discharge.
Energy Density: The energy density of lithium-iron phosphate batteries is usually between 130 and 160 watt-hours per kilogram (Wh/kg).
- c. Lead-acid batteries
-
The lead-acid battery has been mentioned before, but you still have doubts?
What is the difference between AMG and lead-acid batteries?
What is a gel battery?
...
Don't worry, here will give you a clear sort of their differences and similarities.
Lead-acid batteries can be classified into the following types:
Flooded Lead-Acid Batteries: These are the most common type of lead-acid batteries. They have a liquid electrolyte, typically a mixture of water and sulfuric acid, which is free to move within the battery's casing.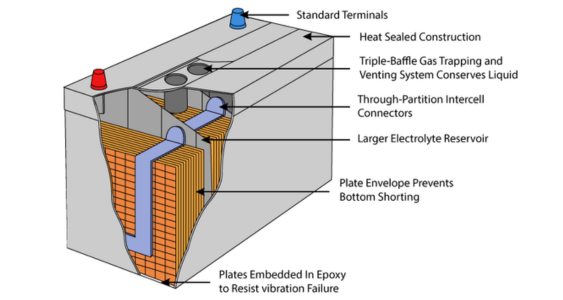
Here are some key characteristics and features of flooded lead-acid batteries:
• Liquid Electrolyte: Flooded batteries contain a liquid electrolyte solution, usually a mixture of water and sulfuric acid. The liquid electrolyte is free to move within the battery's casing.
• Removable Cell Caps: Flooded batteries have removable cell caps that allow for the inspection and maintenance of the electrolyte level and specific gravity. The specific gravity is a measure of the concentration of sulfuric acid in the electrolyte and indicates the battery's state of charge.
• Water Topping: Flooded batteries require periodic maintenance, including the addition of distilled water to maintain the proper electrolyte level. The water evaporates during the charging process, and topping up with distilled water helps prevent the plates from being exposed to the air, which could lead to sulfation.
• Venting System: Due to the production of gases during charging, flooded batteries have a venting system to release the excess gas and prevent the buildup of pressure inside the battery. This venting system requires proper ventilation in the battery installation area.
• Deep Discharge Capability: Flooded batteries are designed to handle deep discharges, making them suitable for applications where occasional heavy loads or long-duration discharges are expected.
• Economical: Flooded lead-acid batteries are generally less expensive compared to other battery technologies, making them a cost-effective choice for various applications.
Flooded lead-acid batteries are commonly used in automotive applications, off-grid renewable energy systems, backup power systems, and in heavy-duty applications where durability and reliability are critical.
Sealed Lead-Acid (SLA) Batteries: Also known as valve-regulated lead-acid (VRLA) batteries, these batteries are designed to be maintenance-free and are sealed to prevent electrolyte leakage. They are further classified into two subtypes:
a.Absorbent Glass Mat (AGM) Batteries: These batteries use a fiberglass mat soaked in electrolyte to absorb and hold the electrolyte within the battery. The mat also acts as a separator between the plates.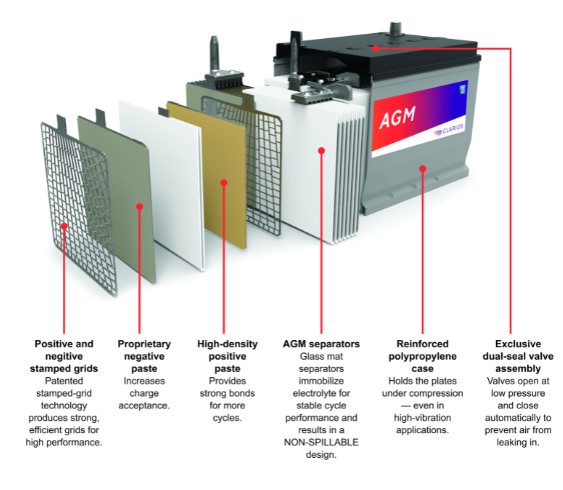
Here are some key points about AGM batteries:
• Construction: AGM batteries consist of lead plates and an electrolyte absorbed within a glass mat separator. The electrolyte is immobilized in the glass mat, making it non-spillable and maintenance-free.
• Operation: AGM batteries work by using a chemical reaction between the lead plates and the electrolyte to produce electricity. The absorbed glass mat separator helps in retaining the electrolyte and provides a large surface area for chemical reactions, resulting in high power density and quick recharge capabilities.
• Sealed and Valve-Regulated: AGM batteries are sealed, which means they do not require water or electrolyte replenishment like traditional flooded lead-acid batteries. They are also valve-regulated, meaning they have a pressure relief valve to vent excess gas and maintain the internal pressure.
• Deep Cycle Capability: AGM batteries are known for their deep cycle capability, which means they can discharge a significant portion of their capacity without being damaged. They are commonly used in applications that require frequent deep discharges and recharges, such as renewable energy systems, electric vehicles, and marine applications.
• Maintenance-Free: AGM batteries are virtually maintenance-free since they do not require regular water additions or electrolyte checks. However, they still require proper charging and storage conditions to maximize their lifespan and performance.
• Advantages: AGM batteries offer several advantages over other battery types. They have a low self-discharge rate, are more resistant to vibration and shock, and can be mounted in various orientations. They also have a faster recharge rate and can provide high current output when needed.
• Applications: AGM batteries are used in a wide range of applications, including backup power systems, uninterruptible power supplies (UPS), alarm systems, medical equipment, recreational vehicles (RVs), off-grid solar systems, and more.
b.Gel Batteries: Gel batteries use a thickening agent, typically silica, to immobilize the electrolyte. This creates a gel-like consistency, which reduces the risk of electrolyte leakage and allows for different orientations of the battery.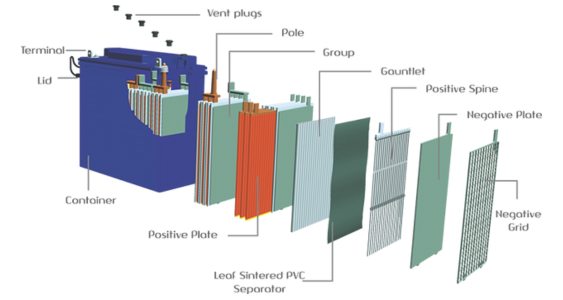
Here's an overview of gel batteries:
• Gel Electrolyte: Gel batteries use a thickened electrolyte in the form of a gel. The electrolyte consists of a sulfuric acid solution mixed with silica to create a gel-like substance. This gel electrolyte immobilizes the acid and prevents it from flowing freely.
• Construction: Gel batteries typically have lead plates, similar to other lead-acid batteries, but with a unique separator material that absorbs and retains the gel electrolyte. The gel electrolyte reduces the risk of acid leakage, making the batteries spill-proof and maintenance-free.
• Deep Cycle Capability: Like AGM batteries, gel batteries are designed for deep cycle applications. They can withstand repeated deep discharges and recharges without significant loss of capacity. This makes them suitable for applications that require frequent cycling, such as renewable energy systems, electric vehicles, and marine applications.
• Sealed and Valve-Regulated: Gel batteries, like AGM batteries, are sealed and valve-regulated. They do not require regular maintenance, such as adding water or checking electrolyte levels. The pressure relief valve allows excess gas to escape and helps maintain the internal pressure of the battery.
• Temperature Sensitivity: Gel batteries have a lower sensitivity to temperature extremes compared to AGM batteries. They perform well in both high and low-temperature environments. The gel electrolyte provides improved thermal stability, making them suitable for applications in extreme climates.
• Vibration and Shock Resistance: Gel batteries are highly resistant to vibration and shock due to the immobilized gel electrolyte. This makes them a preferred choice for applications where the battery may experience frequent movement or mechanical stress.
• Slower Charge Rate: One limitation of gel batteries is their relatively slower charge rate compared to AGM batteries. The gel electrolyte inhibits the movement of ions, resulting in a slower charging process. It's important to use a compatible charger specifically designed for gel batteries to avoid overcharging.
• Applications: Gel batteries are commonly used in various applications, including renewable energy systems, off-grid solar systems, golf carts, electric wheelchairs, scooters, and other mobility devices. They are also preferred in applications where safety, vibration resistance, and deep cycling capability are crucial.
Summary
Although lead-acid batteries still occupy a high market share in the application market due to their low price. But in recent years, with the awakening of people's awareness of environmental protection, more and more people have begun to abandon the polluting lead-acid batteries and replace them with the more environmentally friendly lithium-ion batteries.
- d. Lithium polymer batteries
-
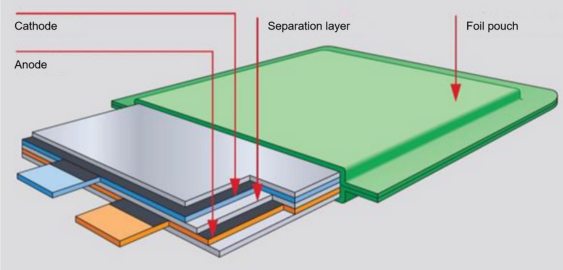
Lithium polymer batteries, also known as Li-Po batteries, are a type of rechargeable battery commonly used in portable electronic devices. They are a variation of lithium-ion batteries and share many similarities but differ in terms of their construction and electrolyte.
Here are some main information about lithium polymer (Li-Po) batteries:
Li-Po batteries use a polymer electrolyte instead of a liquid electrolyte found in traditional lithium-ion batteries. This polymer electrolyte is usually a solid or gel-like substance, which allows for greater flexibility in the battery's form factor. This flexibility makes Li-Po batteries ideal for devices with space constraints or irregular shapes, such as smartphones, tablets, drones, and wearable devices.
• Energy density: Li-Po batteries typically have energy densities ranging from 150 to 200 watt-hours per kilogram (Wh/kg). This high energy density allows for longer battery life and more compact designs compared to other battery technologies.
• Discharge rate: Li-Po batteries are known for their high discharge rates, often exceeding 20C (where C represents the battery's capacity). Some high-performance Li-Po batteries can even handle discharge rates of 50C or higher, enabling them to deliver large amounts of power quickly.
• Cycle life: Li-Po batteries can typically withstand hundreds of charge and discharge cycles before their capacity starts to degrade significantly. A well-maintained Li-Po battery can retain about 80% of its original capacity after 300-500 cycles.
• Self-discharge rate: Li-Po batteries have a relatively low self-discharge rate. They can retain approximately 5-10% of their charge per month when stored at room temperature. This feature makes them suitable for devices that may be idle for extended periods without losing much charge.
• Voltage: Li-Po batteries usually have a nominal voltage of 3.7 volts per cell. However, when fully charged, the voltage can reach around 4.2 volts per cell. It's important to note that Li-Po batteries require specialized chargers designed to handle their voltage and charging characteristics.
• Safety considerations: Li-Po batteries are more sensitive to overcharging, over-discharging, and high temperatures compared to other battery types. If mistreated, they can swell, overheat, or even catch fire or explode. It is crucial to follow safety guidelines, use appropriate chargers, and avoid physical damage to the battery.
- e. Nickel-Metal Hydride Battery
-

Composition and Working Principle:
Nickel-Metal Hydride (NiMH) batteries consist of a positive electrode (nickel hydroxide), a negative electrode (metal hydride), and an electrolyte. During discharge, hydrogen ions from the metal hydride electrode combine with hydroxide ions from the electrolyte, creating water. The electrons released flow through the external circuit, generating electrical energy.
Voltage:
NiMH batteries typically have a nominal voltage of 1.2 volts per cell. Multiple cells can be connected in series to increase the overall voltage.
Capacity and Energy:
NiMH batteries have a capacity rating, measured in ampere-hours (Ah) or milliampere-hours (mAh), which represents the amount of charge the battery can store. The energy capacity of a NiMH battery is determined by multiplying its capacity by the nominal voltage.
Charging and Discharging:
NiMH batteries can be charged using appropriate charging techniques. During charging, a higher voltage is applied to reverse the chemical reactions that occurred during discharge. Discharging involves the release of stored energy as electrical power.
Memory Effect:
NiMH batteries are susceptible to the memory effect, where the battery's capacity is reduced if it is repeatedly charged without being fully discharged first. However, modern NiMH batteries are less prone to this effect compared to earlier versions.
Environmental Impact:
NiMH batteries are more environmentally friendly than some other battery types (such as lead acid battery), as they do not contain toxic heavy metals like lead or cadmium. However, they still require proper disposal or recycling due to the presence of other materials like nickel and metal hydride.
Applications:
NiMH batteries are commonly used in various applications, including portable electronics, hybrid vehicles, cordless power tools, and other high-drain devices. They offer a balance between capacity, energy density, and cost-effectiveness.
- f. Silver-Zinc Battery
-

Composition and Working Principle:
Silver-Zinc (Ag-Zn) batteries consist of a positive electrode (silver oxide, Ag2O), a negative electrode (zinc, Zn), and an alkaline electrolyte. During discharge, the silver oxide electrode reduces to form silver (Ag) and releases hydroxide ions (OH-) into the electrolyte. Simultaneously, the zinc electrode oxidizes, dissolving into zinc ions (Zn2+) and generating electrons (e-). The overall reaction can be represented as: 2Ag2O + Zn -> 4Ag + ZnO
Voltage:
Silver-Zinc batteries typically have a nominal voltage of 1.6 to 1.9 volts per cell.
Capacity and Energy:
Silver-Zinc batteries have a relatively high energy density of around 100-120 Wh/kg. They offer a capacity ranging from 150 to 500 mAh per cell.
Charging and Discharging:
During charging, the reactions are reversed. Silver is oxidized back to silver oxide on the positive electrode, and zinc is plated back onto the negative electrode.
Advantages:
Silver-Zinc batteries offer several advantages, including high energy density, longer cycle life (typically over 500 cycles), and relatively low environmental impact. They are also considered safer compared to some other battery chemistries.
Limitations:
One limitation of Silver-Zinc batteries is the potential for the formation of silver dendrites, which can cause internal short circuits and reduce the battery's performance over time. Careful charging and discharging procedures are necessary to minimize dendrite formation.
Applications:
Silver-Zinc batteries are used in various applications, such as military equipment, medical devices, hearing aids, and aerospace applications. Their high energy density and reliability make them suitable for demanding and high-performance applications.
- g. Lead-Carbon Battery
-
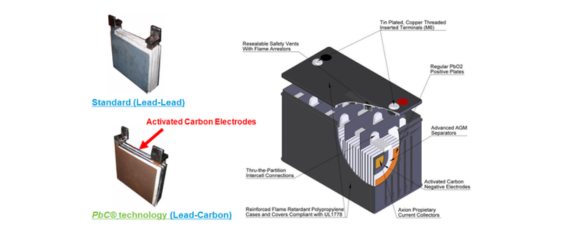
Composition and Working Principle:
Lead-Carbon batteries combine a positive electrode of lead dioxide (PbO2) and a negative electrode containing carbon materials. During discharge, the lead dioxide electrode converts to lead sulfate (PbSO4), while the carbon electrode absorbs and releases ions. This process generates electrical energy. During charging, the reactions are reversed, converting lead sulfate back to lead dioxide and restoring the carbon electrode.
Voltage:
Lead-Carbon batteries typically have a nominal voltage of 2 volts per cell.
Capacity and Energy:
Lead-Carbon batteries have a capacity rating ranging from approximately 40 Ah to 200 Ah per cell, depending on the battery size and design. The energy capacity is determined by multiplying the capacity by the nominal voltage.
Charging and Discharging:
Lead-Carbon batteries can be charged using appropriate charging techniques. During charging, a voltage higher than the battery voltage is applied to convert lead sulfate back into lead dioxide and to replenish the carbon electrode. Discharging involves the release of stored energy as electrical power.
Advantages:
Lead-Carbon batteries offer several advantages over traditional lead-acid batteries, including improved cycle life (typically over 2,000 cycles), higher charge acceptance, and better performance in partial state of charge (PSoC) conditions. The addition of carbon to the negative electrode enhances the battery's ability to handle high-current and high-rate applications.
Applications:
Lead-Carbon batteries find applications in renewable energy storage systems, hybrid electric vehicles (HEVs), backup power systems, and other industrial applications. They are particularly suitable for applications requiring frequent cycling, high charge and discharge rates, and long-term reliability.
Environmental Impact:
Lead-Carbon batteries have reduced lead content compared to conventional lead-acid batteries, leading to improved environmental impact. They also exhibit better cycling capability, resulting in longer service life and reduced waste generation.
- h. Sodium-Sulfur Battery
-
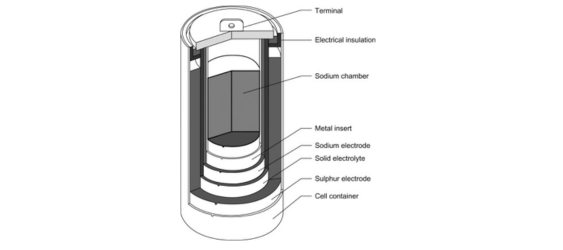
Composition and Working Principle:
Sodium-Sulfur (NaS) batteries consist of a solid-state electrolyte, a sodium (Na) positive electrode, and a sulfur (S) negative electrode. The working principle involves the reversible redox reactions between sodium and sulfur. During discharge, sodium ions (Na+) migrate from the positive electrode through the electrolyte to the negative electrode, where they react with sulfur to form sodium polysulfides. This process releases electrical energy. During charging, the reactions are reversed, converting the sodium polysulfides back to sodium ions and sulfur.
Voltage:
Sodium-Sulfur batteries typically have a nominal voltage of 2 volts per cell.
Capacity and Energy:
Sodium-Sulfur batteries have a high energy density, ranging from 100 Wh/kg to 200 Wh/kg. The capacity is usually in the range of 200 to 500 ampere-hours (Ah) per cell.
Operating Temperature:
Sodium-Sulfur batteries operate at high temperatures, typically around 300 to 350 degrees Celsius (572 to 662 degrees Fahrenheit), to facilitate the mobility of sodium ions and enhance the electrochemical reactions.
Charging and Discharging:
Sodium-Sulfur batteries require careful temperature control during charging and discharging to maintain their performance and prevent safety issues. Charging involves applying a higher voltage to drive the sodium ions back to the positive electrode, while discharging involves the release of stored energy as electrical power.
Advantages:
Sodium-Sulfur batteries offer several advantages, including high energy density, long cycle life (over 3,000 cycles), and excellent charge/discharge efficiency. They are suitable for applications requiring large-scale energy storage, such as grid-level energy storage systems.
Applications:
Sodium-Sulfur batteries are used in various applications, including renewable energy storage, electric grid stabilization, and off-grid power systems. They are particularly well-suited for applications that require long-duration energy storage and high power output.
- j. Nickel-Iron Battery
-
Composition and Working Principle:
Sodium-Ion batteries consist of a sodium-based positive electrode, a carbon-based negative electrode, and a sodium-ion-conducting electrolyte. The working principle involves the reversible intercalation/deintercalation of sodium ions (Na+) into/from the electrode materials. During discharge, sodium ions migrate from the positive electrode to the negative electrode through the electrolyte, creating a flow of electrons that generates electrical energy. During charging, the sodium ions are driven back to the positive electrode.
Voltage:
Sodium-Ion batteries typically have a nominal voltage of 3.7 to 4 volts per cell.
Capacity and Energy:
Sodium-Ion batteries have a capacity rating typically ranging from 100 to 150 milliampere-hours per gram (mAh/g) for the electrode materials. The energy density can range from 100 to 150 watt-hours per kilogram (Wh/kg).
Charging and Discharging:
Sodium-Ion batteries can be charged using appropriate charging techniques. During charging, a higher voltage is applied to drive the sodium ions back to the positive electrode. Discharging involves the release of stored energy as electrical power.
Advantages:
Sodium-Ion batteries offer several advantages, including the abundance and low cost of sodium compared to lithium, which makes them potentially more cost-effective. They also have a long cycle life, improved safety compared to lithium-ion batteries, and are more environmentally friendly.
Applications:
Sodium-Ion batteries are being explored for various applications, including large-scale energy storage systems, renewable energy integration, and grid stabilization. They have the potential to be used in electric vehicles, portable electronics, and other energy storage applications.