Isikhokelo sokugqibela kwiibhetri
Esi sikhokelo sinolwazi, ke nceda ufumane into ofuna ukuyifunda kwitheyibhile yeziqulatho ngokuxhomekeke kwinqanaba lakho lolwazi lwebhetri.Ewe kunjalo ukuba uyi-qala, ke nceda uqalise ekuqaleni.
Ingcebiso yangaphambi kokufunda: Cofa kube kanye kwibhokisi yetekisi kunye nesicatshulwa esineenkcukacha siya kwanda;Cofa kwakhona kwaye isicatshulwa esineenkcukacha siyafihla.
Intshayelelo
- Ukubaluleka kunye nokusetyenziswa okuxhaphakileyo kweebhetri.
-
Iibhetri zibaluleke kakhulu kuluntu lwanamhlanje kwaye zisetyenziswa kuluhlu olubanzi lwezicelo (ngokuphuhliswa kwetekhnoloji, ezinye kwaye ezinye ziguqulwa zibe ngamandla ebhetri).Babonelela ngezisombululo zamandla ezikhoyo, ezihlaziyiweyo nezingxamisekileyo eziqhuba uphuhliso lwetekhnoloji, ukusetyenziswa kwamandla okuzinzileyo kunye nenkqubela phambili kuluhlu olubanzi lwamashishini.

1. Izixhobo ze-elektroniki eziphathwayo: Iifowuni eziphathwayo, iitafile, iilaptops kunye neekhamera zedijithali.
2. Ukuthutha: Izithuthi zombane kunye ne-hybrid zisebenzisa iibhetri njengesixhobo sokugcina amandla.Ngemfuno eyandisiweyo yamandla ahlaziyekayo kunye neendlela ezinobuhlobo bendalo, iibhetri zidlala indima ephambili ekuqhubeni uphuhliso oluzinzileyo lwezothutho.
3. Ukugcina amandla ahlaziyiweyo: Iibhetri zisetyenziselwa ukugcinwa kwemithombo yamandla ahlaziyiweyo efana neSolar kunye nePhando lomoya.Ngokugcina amandla ombane kwiibhetri, banokubonelela ngombane oqinileyo xa i-solar okanye amandla omoya ayifumaneki.
4. Amandla kaNgxamisekileyo: Iibhetri zidlala indima ebalulekileyo njengomthombo wamandla obuva umva kwiimeko zongxamiseko.Umzekelo, izixhobo ezinje ngeefowuni ezingenantambo, iitotshi kunye nezibane ezingxamisekileyo zifuna iibhetri ukubonelela ngamandla athembekileyo.
5. Izixhobo zonyango: Uninzi lwezixhobo zonyango, ezinjenge-pacemakers kunye ne-ventilators ye-offacial, sebenzisa iibhetri njengomthombo wamandla.Uzinzo kunye nokuthembeka kweebhetri kubalulekile ekusebenzeni kwezi zixhobo zibaluleke kakhulu.
6. Izicelo zomkhosi: Iibhetri zisetyenziswa kuluhlu olubanzi lwezicelo zomkhosi, ezinje ngezixhobo zonxibelelwano zomkhosi, iinkqubo zokuhamba kunye needrones.Iibhetri zinokubonelela ngoncedo lwamandla azimeleyo kwaye luphucule amandla omdibaniso kwibala ledabi.
7. Ishishini: Iibhetri zisetyenziswa kumzi-mveliso weenkqubo zebhetri, ubonelelo ngoncedo olungxamisekileyo kunye neenzwa ngaphandle kwentambo.Banika amandla anokuthenjwa kwaye baqinisekise ukuqhubeka nokukhuseleka kwemveliso yemveliso.
- Isishwankathelo semigaqo esisiseko kunye neendlela zokusebenza zebhetri.
-
Umlingo weebhetri buxhomekeke kubuchule babo bokuguqula amandla amachiza kumandla ombane.Ibhetri inama i-electrode ezimbini (ezihle nezimbi) kunye ne-electrolyte.I-electrolyte isebenza njengomqhubi we-ion, evumela impendulo yemichiza phakathi kwe-electrode.
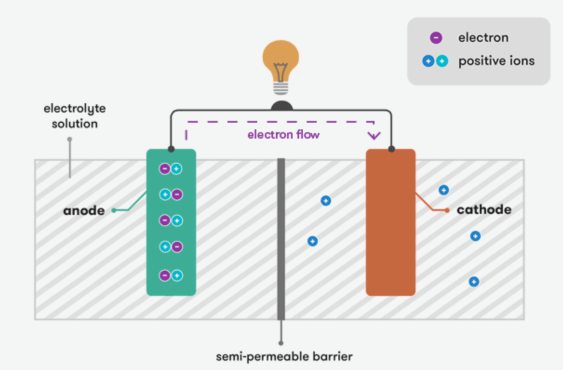
Umgaqo osisiseko webhetri usekwe kwi-elektroniki yokuphendula.Xa impendulo yekhemikhali yenzeka, kuvelisa ukuhamba kwee-elektroni.Kwimeko efudukayo, ibhetri igcina iichemicals eziphakathi kwe-electrodes elungileyo nengalunganga, kwaye impendulo yekhemikhali iguqukele.Xa ibhetri iqhagamshelwe kwisekethe yangaphandle, impendulo yekhemikhali iqala, ebangela imichiza kwindawo elungileyo yokufikelela kwi-oxiving kunye nekhemikhali kwindawo engalunganga yokunciphisa.Ngenxa yoko, ukuphuma kwee-elektroni ukusuka kwi-terminal engalunganga kwi-terminal elungileyo, evelisa umbane wangoku.Le nkqubo iyaqhubeka kude kuphele iichemicals.
Iindidi ezahlukeneyo zeebhetri zisebenzisa ukuphendula okwahlukileyo kweekhemikhali zokuvelisa umbane.Umzekelo, olona hlobo luqhelekileyo lwebhetri ye-lithium-ion: I-electrode yayo yenziwa nge-lithium ye-libalt (njenge-cobabax ye-bobalt oxide okanye i-lelium ye-lilium phosphate) kunye ne-electrode ye-phosphate ye-carbon (ezinje ngegrafu).Kwimeko etyunjiweyo, i-lithium i-ion ingena kwi-electrode eyakhayo kwizinto ezimbi.Ngexesha lokukhupha, i-lithium i-i-i-i-i-i-ifakwe kwi-electrode engalunganga kwaye ibuyele kwi-electrode eyakhayo, ukukhulula ii-elektroni.
- Ixabiso lokuba nesikhokelo sokugqibela kwiibhetri kubafundi.
-
Isikhokelo sokugqibela sibalulekile kumfundi ngenxa yezizathu ezininzi:
1. Ukubonelela ngolwazi oluchanekileyo: I-Intanethi igcwele iziqwenga zolwazi kunye nezimvo ezingqubanayo.Isikhokelo sokugqibela sinika ulwazi oluchanekileyo noluchanekileyo ngokudibanisa nokudibanisa imithombo ethembekileyo ukunceda abafundi ukuba bafumane ulwazi abalufunayo kwaye baphephe ulwazi olulahlekisayo okanye ulwazi olungalunganga.
2. Gcina ixesha kunye nomzamo: Ukukhangela i-Intanethi ngezihloko ezithile zihlala zifuna ixesha elininzi lokuqhubela phambili nokuqinisekisa ukuthembeka kolwazi.Isikhokelo sokugqibela sigcina ixesha kunye nomzamo ngokuzisa ulwazi oluchanekileyo ukuze abafundi bafumane lonke ulwazi abaludingayo kwindawo enye.
3. Ukucombulula iingxaki kunye nokudideka: I-Intanethi ihlala ibonisa iimpendulo ezahlukeneyo kumbuzo ofanayo okanye ukuphikisana phakathi kolwazi.Esona sikhokelo sinceda abafundi ukuba balibale ukudideka kunye nokuthatyathwa yimbono eyahlukileyo kunye nemithombo yolwazi egunyazisiweyo ukunika ezona mpendulo zinokuthenjwa.
4. Nika isikhokelo kunye nengcebisoIsikhokelo sokugqibela asinikeli nje iinyani kunye nolwazi, kodwa zinokubonelela ngesikhokelo nengcebiso.
- Iindidi ezahlukeneyo zeebhetri: Imigaqo, iimpawu, kunye nokusetyenziswa.
-
Nazi ezinye zeentlobo ezi-5 eziqhelekileyo zeebhetri, kubandakanya imigaqo, iimpawu kunye nokusetyenziswa.Ukuba ufuna ulwazi olubanzi kwiindidi zebhetri, unokutsiba eli candelo kwaye uye ngqo kwiindidi zesicelo kunye nokusetyenziswa "ngezantsi.
Iibhetri ze-acid
•Umgaqo: Iibhetri ze-Acid-I-Acid Sebenzisa indlela yokuphendula yemichiza phakathi kokukhokela kwaye ikhokele diokside yokuvelisa amandla ombane.
•Iimpawu: Iindleko eziphantsi, ukuqala okuphakathi kunye nokuxinana kwamandla, kodwa zikhulu kwaye zinzima.
•Izicelo: Iibhetri ze-AutoMotive Bitter, i-UPS (i-Investracity), njl.
I-Li-ion (i-lithium-ion)
•Umgaqo: Ibhetri ye-lithium-ion Sebenzisa ukufuduka kwe-lithium ze-lithium ze-ion phakathi kwe-electros elungileyo nengalunganga ukugcina nokukhupha amandla ombane.
•Iimpawu: Ukuxinana kwamandla aphezulu, ubunzima bomzimba kunye nobude obude bobomi.Ukurhoxisa okuphezulu kunye nokuhlisela ukusebenza kakuhle.
•Izicelo: Izixhobo zefowuni (i.G. Iifowuni zefowuni, iikhompyuter zetafile), izixhobo ze-elektroniki eziphathwayo kunye nezithuthi zombane.
I-NICDE (i-nickel-cadmium)
•Umgaqo: Iibhetri ze-nided zivelisa amandla ombane ngokusabela kwi-NCECEL kunye ne-CADMRDROID Hydroxide.
•Iimpawu: Iziphumo eziphezulu kunye nobomi obude, kodwa ziqulathe i-cadmium enengozi enkulu, enefuthe elithile kwindalo esingqongileyo.
•Izicelo: Iikhamera zedijithali, izixhobo eziphathwayo kunye ne-drones, njl.
I-Nimh (i-nickel-nyibili) ye-hydride hidride
• Umgaqo: Ibhetri ye-NIMH isebenzisa indlela yokuphendula yekhemikhali phakathi kwe-NCECEL kunye ne-hydrogen ukugcina nokukhupha amandla ombane.
•Iimpawu: Ukuxinana kwamandla aphezulu, ubomi obude, akukho longcoliseko kunye nokusebenza okuphezulu kobushushu obuphezulu.
•Izicelo: Izithuthi zeHybrid, iinkqubo zokugcina zamandla, njl. Njl.
Ibhetri ye-lipo (i-lithium polymer) ibhetri

•Umgaqo: Ibhetri ye-lithium polymer iyafana nebhetri ye-lithium ye-ion, kodwa isebenzisa i-polymer eqinileyo endaweni ye-elektrolyte yolwelo.
•Iimpawu: Ukuxinana kwamandla aphezulu, ubunzima obukhulu, ukhuseleko olungcono kunye nenqanaba lokuzibandakanya.Ilungele izixhobo ezincinci.
•Izicelo: Iilaptops, iiwotshi ze-Smart kunye nezixhobo zonyango eziphathwayo njlnjl.
- Ulwazi lwe-physics yeebhetri
- Ivolthi (v):
I-voltage imele umahluko onokubakho onokubakho phakathi kwamanqaku amabini kwisekethe.Ilinganiswa kwiVolts (v).I-voltage ibhetri kwibhetri iqhele i-v_battt.
Intlawulo (q):
Intlawulo ibhekisa kwinani lentlawulo yombane egcinwe ebhetri.Ilinganiswa kwi-coulombs (c) okanye iiyure ze-AMPERE) (Ah).Ubudlelwane obuphakathi kwentlawulo kunye nenqanaba elinikiweyo: Intlawulo (q) = umthamo (c) voltage (v)
Umthamo (c):
Umthamo umele inani lebhetri.Ihlala ilinganiswa kwi-Amprere-tere-yure (ah) okanye iiyure ze-milliampere (Mah).Ubudlelwane phakathi komthamo, intlawulo, kwaye amandla anikezwe ngu: Amandla (e) = umthamo (c) × voltage (v)
Amandla (e):
Amandla ngamandla okwenza umsebenzi okanye amandla enkqubo yokubangela utshintsho.Kwimeko yeebhetri, amandla ahlala alinganiswa kwiiyure ze-Watt (wh) okanye uJoules (J).Ulwalamano phakathi kwamandla, amandla, kunye nentlawulo enikezwe: Amandla (e) = ntlawulo (q) vottage (v)
Amandla (p):
Amandla amele umlinganiso apho umsebenzi owenziweyo okanye amandla adluliselwa khona.Ilinganisa kwii-watts (w).Amandla kwisekethe ibalwa kusetyenziswa ifomula: Amandla (p) = i-voltage (v) × (i)
Uqhagamshelo:
1. Xa iibhetri zixhunyiwe kothotho, i-voltage iyonke kwisekethe yesekethe yebhetri.Okwangoku ihlala ifana.
I-voltage iyonke (v_total) = v1 + v2 + v3 + ...
2. Xa iibhetri ziqhagamshelwe kuphuculo, amandla ewonke sisixa sesakhono sebhetri.Kungenxa yokuba ikho ngoku ihlala ifana, kodwa ivolthi iyonke iyanda.
Iyonke i-STATAL (C_total) = C1 + C2 + C3 + ...
Unxibelelwano olufanayo:
1. Xa iibhetri ziqhagamshelwe ngokuhambelana, i-voltage iyonke ihlala ifana nebhetri yomntu ngamnye, ngelixa iyonke iyonke inesixa se-curpores ehamba ebhetri nganye.
Iyonke yangoku (i_total) = i1 + i3 + i3 + ...
2. Xa iibhetri zixhunyiwe kwi-parallel, umthamo opheleleyo ulingana nesikhundla sebhetri enye.Kungenxa yokuba i-voltage ihlala ifana, kodwa iyonke iyonke.
I-Assali iyonke (C_total) = C1 = c2 = c3 = ...
- Imigaqo yebhetri eqhelekileyo kunye neenkcazo.
-
1. Umthamo webhetriIxabiso lamandla ombane apho ibhetri inokugcina khona, ihlala ibonakaliswa kwiiyure ze-AMP (AH) okanye i-milli-amps (Mah).
2. I-Voltage: Umahluko onokubakho okanye umahluko we-voltage yebhetri, eboniswe kwi-Volts V. imele inani lamandla ombane ibhetri.
3. Iseli yebhetri: Iseli nganye kwibhetri, equkethe i-electrode elungileyo, i-electrode ne-electrolyte.
4. Ipakethi yebhetri: Ukuphuhliswa kweeseli ezininzi zebhetri zidibene.Zihlala zixhunyiwe kwaye zilawulwa ngabasebenzi, iibhodi zesekethe kunye nezinye izinto.
5. Uqhagamshelo: Iiseli ezininzi zebhetri ezixhunyiwe ngokulandelelana, kunye ne-terminal elungileyo exhunyiwe kwi-terminal engalunganga, ukwandisa i-voltage iyonke.Xa uqhagamshelwe kungcelele, i-voltages yeseli iyasuswa.
6. Unxibelelwano olufanayo: Qhagamshela iiseli ezininzi zebhetri ngokulandelelana, kunye ne-terminal elungileyo exhunyiwe kwi-terminal engalunganga, ukwandisa amandla akho okuqala kunye nomthamo.Xa uqhagamshelwe ngokuhambelana, amandla eeseli zebhetri zongezwa kunye.
7. Shaja: Ukondla amandla ombane kwibhetri ukusuka kumthombo wangaphandle ukubuyisela amandla emichiza agcinwe kwibhetri.
8. Ukuqhuba: Ukukhutshwa kwamandla ombane kwibhetri yokusetyenziswa ekuboneleleni izixhobo ze-elektroniki okanye kwisekethe.
9. Umjikelo wokuTyaIbhekisa kwinkcitho epheleleyo yokutshaja kunye nokukhupha inkqubo.
10. Ukubiza impumelelo: Umyinge ophakathi kwamandla ombane athathe ibhetri kunye namandla ombane agcinwe ngexesha lenkqubo yokutshaja.
11. Ukuzikhupha: Ixabiso apho ibhetri iphulukana namandla ngokwayo xa engasebenzi.
12. Ubomi bebhetri: Ixesha lokuphila kwebhetri, lihlala lilinganiswe ngokwenani lemijikelezo ehlawulwayo okanye ixesha lokusebenzisa.
13. Ubomi bebhetriIxabiso lexesha ibhetri linokuqhubeka nokubonelela ngamandla emva kwentlawulo.
14. Ukutshaja ngokukhawulezaItekhnoloji yokutshaja ehambisa amandla kwibhetri ngokukhawuleza ukunciphisa ixesha lokutshaja.
15. Inkqubo yoLawulo lwebhetri (ii-BMs)Inkqubo yombane ebeka iliso kwaye ilawula imeko yebhetri, ukutshaja kunye nokukhuthaza inkqubo yokukhuphela kwaye ikhusela ibhetri kwiimeko ezimbi ezifana ne-overdischarcage.
16. Ibhetri yobomi: Inani lemijikelezo ethambileyo ibhetri inokuthi igqibe, ihlala ilinganiswa ngokutshaja nasekukhutshelweni kwilahleko ethile enjengama-80%.
17. Inqanaba lemigangatho yexabiso: Elona nqanaba liphezulu lamaxabiso anokwamkelwa ngokukhuselekileyo yibhetri, eboniswe njengomyinge wetyala elityala.
18. Ubuninzi bexabiso lokuphulukanaIreyithi ephezulu yangoku apho ibhetri inokukhutshelwa ngokukhuselekileyo, ibonakaliswe njengomlinganiselo womthamo wangoku.
19. Isekethe yokukhusela ibhetri: Isixhobo sokhuseleko esisetyenziselwa ukubeka iliso kwimeko yebhetri kunye nokukhupha isekethe yebhetri kwimeko yokugqitha ngaphezulu, i-overdischarcar, i-overmature, i-overmature, ingozi kwibhetri.
20. Ibhetri yebhetri: Umahluko kunye nokuchongwa phakathi kweendawo ezilungileyo nezingalunganga zebhetri, zihlala ziboniswa ziimpawu + kwaye - okanye amanqaku.
21. Ukurisayikilisha IbhetriInkqubo yokulahleka kweebhetri ezisetyenzisiweyo ukuze iphinde ifumane kwaye ilahle izixhobo ezinobungozi eziqulathwe kuzo kunye nokuphinda isebenzise izixhobo ezinokuphinda zisebenzise.
22. Ukukhutshwa okunzuluImeko apho ibhetri ikhutshiwe kwinqanaba eliphantsi kakhulu okanye liphelile ngokupheleleyo.Ukukhutshwa okunzulu akusenziwa rhoqo ukuphepha iziphumo ezingalunganga kubomi bebhetri.
23. Ukukhutshwa okukhawulezayoIcebo lokukhupha elikhupha amandla ebhetri ngexesha eliphezulu lexesha elifutshane.
24. Ukusilela kwebhetriImeko apho ibhetri ayikwazi ukubonelela ngamandla awoneleyo okanye ukugcina ukusebenza kwesiqhelo, okunokubangelwa zizizathu ezahlukeneyo ezinjengokuphanziswa okanye ukonakala.
25. I-thermal ebalekayo Ibhekisa kunyuka okukhawulezileyo kunye nokungalawuleki kwiqondo lebhetri phantsi kweemeko ezingaqhelekanga, njengokugqitha ngaphezulu, okungaphezulu, okunokubangela ukuba ibhetri iqhume okanye ibambe umlilo.
26. Ibhetri ye-electrodes: I-Electrodes elungileyo nengalunganga kwibhetri, ezingamacandelo aphambili zokugcina kunye nokukhupha intlawulo yombane.
27. Isikhululo sebhetri: Indawo okanye inkonzo yokutshintshwa ngokukhawuleza kweebhetri kwizithuthi zombane zokubonelela uluhlu olude.
28. Impendulo ye-electrochemical: Impendulo yekhemikhali eyenzeka kwibhetri ukuguqula amandla eekhemikhali kumandla ombane ngenkqubo ye-reox.
29. Electrolyte: Ulwelo oluqinileyo okanye oluqinileyo olusetyenziselwa ukuhambisa ii-Ion phakathi kwe-electrods elungileyo nengalunganga yebhetri ukulungiselela ukuphendula kwe-elektroniki.
30. Itshaja: Isixhobo sokudlulisela amandla ombane kwibhetri ukubuyisela amandla awo emichiza.
31. Ukulungelelaniswa kwebhetriInkqubo apho intlawulo okanye ireyithi yokukhupha iseli nganye kwipakethi yebhetri ihlengahlengiswa ukuqinisekisa ukuba intlawulo ilungelelene phakathi kweseli nganye.
32. Ibhetri yangaphandleIyunithi yebhetri esuswayo enokuqhagamshelwa kwisixhobo se-elektroniki ukubonelela ngamandla.
33. Isalathisi sebhetri: Isalathiso okanye isibonisi esibonisa imeko yentlawulo okanye inqanaba lebhetri.
34. Isiphumo seMeko yebhetriI-phenomenon apho isikhundla sebhetri ngokuthe ngcembe sincipha njengoko intlawulo kunye nokugada imijikelezo iyaphindwa, njengoko ibhetri ikhumbula intlawulo encinci kwaye ikhuphe i-ranges encinci.
35. UkunyanzelaIbhekisa kuxhathiso lwangaphakathi lwebhetri, echaphazela ukusebenza kwamandla kunye nokusebenza.
36. Ukhuseleko lobushushu: Umsebenzi okanye isixhobo esibeka iliso kwaye silawula ubushushu bebhetri ukuthintela ukonakala okungapheliyo ukuba iqondo lobushushu liphezulu kakhulu.
37. Ukukhusela i-voltage ephantsiIndlela yokukhusela enqumama ngokuzenzekelayo isekethe ukuthintela ukuqhutywa ngaphezulu xa i-voltage yebhetri iphosa ngaphantsi komqobo okhuselekileyo.
38. Ukhuseleko olugqithisileyoIndlela yokukhusela enqumama ngokuzenzekelayo isekethe ukuthintela ukuqhubela phambili xa intlawulo yebhetri ifikelela kumgangatho wokhuseleko.
39. Ukugcinwa kwebhetriInkqubo yokugcina ibhetri kwimo engasetyenziswanga ixesha elide, ngokufuthi ifuna amanyathelo afanelekileyo ukunciphisa ukukhulula nokukhusela ibhetri.
40. Inkqubo yoLawulo lwebhetri (ii-BMs)Inkqubo ye-elektroniki yokubeka iliso, ukulawula nokukhusela imeko kunye nokusebenza kwepakethi yebhetri, kubandakanya nolawulo lwangoku, i-voltage, ubushushu kunye nezinye iiparamitha.
41. Isalathiso senqanaba lebhetri: Isixhobo okanye umsebenzi obonisa inqanaba lentlawulo esele kwibhetri, idla ngokuvakaliswa njengepesenti okanye ngamanqanaba aliqela.
42. Ixesha lokutshajaIxesha elifunekayo lokuzisa ibhetri ukusuka kwintlawulo ephantsi ukuya kwityala elipheleleyo, eliphenjelelwa ngamandla etshaja kunye nobungakanani bebhetri.
43. Iqondo lokushisa elineqondo: Ubudlelwane phakathi kokusebenza kwebhetri kunye notshintsho kubushushu, olunokuchaphazela umthamo, ukuxhathisa ngaphakathi kunye nokuhlawula / ukukhulula iimpawu zebhetri.
44. Iwaranti yebhetri: Iwaranti yomenzi kwintsebenzo kunye nomgangatho webhetri kangangexesha elithile, idla ngokuchazwa kwiinyanga okanye iminyaka.
45. Isiskhululo sokugcwalisa: Izixhobo okanye indawo esetyenziselwa ukubonelela ngezithuthi zombane okanye ezinye izixhobo zebhetri ukutshaja.
46. Ibhetri: Isixhobo okanye isixhobo esisetyenziselwa ukulinganisa i-voltage, amandla, ukumelana ngaphakathi kunye nezinye iiparamitha zebhetri ukuvavanya impilo kunye nokusebenza kwayo.
47. Ukulinganisa okusebenzayoIndlela yokulawulwa kwebhetri elawula intlawulo kwipakethi yebhetri ngokulawula intlawulo kunye nokugada amaxabiso phakathi kweeseli zomntu ngamnye.
48. UkulinganisaIndlela yolawulo lwebhetri apho intlawulo kwipakethi yebhetri ilungelelaniswe ngokuthintela abaphengululi okanye intlawulo yokuhlawula, ihlala ingasebenzi kakuhle kunokulingana okusebenzayo.
49. Ukupakishwa kwebhetri : Ukupakishwa kwangaphandle kwebhetri, isetyenziselwa ukukhusela iseli, ukubonelela ngenkxaso yolwakhiwo kunye nokukhusela imijikelezo emifutshane.
50. Ukuxinana kwamandla aphezulu: Elona nani liphezulu lamandla ombane anokugcina ibhetri nganye ivolumu okanye ubunzima, ebonisa ukusebenza kwempumelelo yebhetri.
I-51. Ixabiso lokuzithoba eliphantsi: Ireyithi apho ibhetri ilahlekelwa khona amandla ombane ngokwayo icotha kakhulu kwaye igcina imeko enkulu yokuhlawula xa igcinwe okanye ingasetyenziswanga ixesha elide.
52. Ibhetri: Kubhekisa kutshintsho lwezinto ezibonakalayo kumphezulu we-electros xa kutshaja kunye nokukhupha ngenxa yokuphendula kweekhemikhali kwi-elektroniki.
53. Ibhetri ye-electrolyte ukuvuza: Imeko apho i-electrolyte ivuza ibhetri kwindalo yangaphandle, eya kukhokelela ekulahlekisweni kokusebenza kwebhetri okanye ezinye iingxaki zokhuseleko.
54. Inkqubo yokupholisa ibhetriInkqubo esetyenziselwa ukulawula ubushushu bebhetri, nokuba kukutya kobushushu, i-fan okanye i-fan okanye ulwelo olupholayo ukugcina ibhetri ngaphakathi kuluhlu olufanelekileyo lobushushu.
55. Inkqubo yokufudumeza ibhetriInkqubo esetyenziselwa ukubonelela ngobushushu kwibhetri kwimigca yobushushu ephantsi yokuqinisekisa ukusebenza ngokuchanekileyo kwebhetri kumaqondo obushushu asezantsi.
56. Ibhetri ephezulu yokulahla inkunkumaIbhetri ekwaziyo ukuhambisa amandla ombane aphezulu ngoku kwizicelo ezineemfuno eziphezulu zamandla ezinje ngezixhobo zamandla kunye nezithuthi zombane.
57. Ibhetri yesibiniIbhetri enokuthi iphinde iphinde iphinde ibethe ibhetri engenakuphikiswa engakwazi kwakhona.
58. Ibhetri iliso: Isixhobo okanye inkqubo yokubeka iliso kwimeko, i-voltage, ubushushu kunye nezinye iiparamitha zebhetri ngexesha lokwenyani ukubonelela ngolwazi kwaye khusela ibhetri.
- Ulwakhiwo lwebhetri: i-electrode, electrolyte, kunye nesahluli.
-
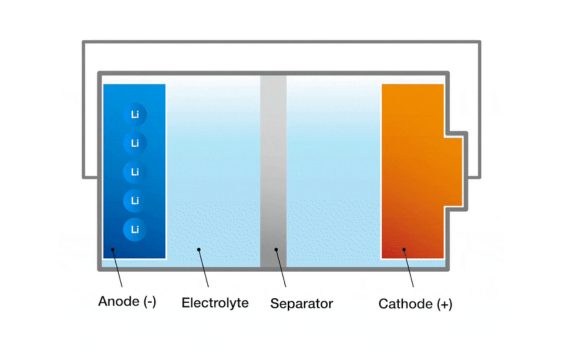
1. Electrodes: I-electrodes kwibhetri yahlulwe yaba yinto engekhoyo ye-electrode.I-electrode eyakhayo kulapho impendulo ye-oxidation yenzeka khona kwibhetri kunye ne-electrode engalunganga apho ikhoyo yokuphendula yenzeka kwibhetri.I-Electrodes elungileyo nengalunganga zenziwe ngezinto ezisebenzayo, ziqhele iintsimbi, i-carbon okanye imichiza isetyenzisiwe.Umahluko onokubakho phakathi kwe-electros elungileyo nengalunganga ivelisa i-voltage yeseli yebhetri.
2. Electrolyte: I-electrolyte yindawo ephakathi phakathi kwe-electrodes evumela i-ions ukuba idlule phakathi kwe-electrode kwaye igcine ibhalansi.I-electrolyte inokuba kwi-wolter, ifomu eqinileyo okanye yejeli, kuxhomekeka kuhlobo lweseli.Kwiseli engamalwelo, i-electrolyte ihlala i-Ionic ye-Ionic ye-IIIC inyibilikisiwe kwisisombululo.
3. DiaphragmI-Diaphragm ngumqobo obonakalayo phakathi kwe-Electros elungileyo nengalunganga, ethintela ukuhamba kwe-elektroni kodwa kuvumela ii-ions ukuba zidlule.Umsebenzi we-diaphragm kukuthintela ukujikeleza okufutshane kwezinto ezilungileyo nezingalunganga ngelixa uvumela i-ion ukuba ihambe ngokukhululekileyo nge-electrolyte kwaye igcina ibhalansi yeseli.Idayaphragm ihlala yenziwe ngezinto ze-polymeric okanye izinto ze-ceramic.
Ezi macandelo zisebenza kunye ukwenza isakhiwo seseli yebhetri.
- Intlawulo kunye nokulahla iinkqubo kwiibhetri: Ukuphendula kwekhemikhali kunye nokuhamba kwangoku.
-
1. Inkqubo yokukhupha: Xa ibhetri ikhutshiwe, amandla emichiza aguqulwa abe ngamandla ombane.Ngexesha lokuphelisa, indlela yokuphendula ye-oxidation yenzeka kwindawo efanelekileyo yesiphelo kunye nendlela yokunciphisa kwi-terminal engalunganga.Impendulo yekhemikhali ivelise ii-elektroni kunye nee-ion.I-electrode elungileyo ikhuphe ii-elektroni, ezihamba ngesekethe yangaphandle ukuvelisa umbane wangoku.I-electrode engalunganga ifumana ii-elektroni, ezidibanisa ii-ion ukwenza imiqathanga.Kwangelo xesha, i-Ions ihamba kwi-electrolyte, igcina ibhalansi yebhetri.
2.Inkqubo yokubiza: Ngexesha lokutshaja kwebhetri, amandla ombane aguqulwa abe ngamandla emichiza ukuze agcine amandla.Ngexesha lenkqubo yokutshaja, umthombo wamandla wangaphandle usebenzisa i-voltage ngaphambili, ibangele ukuba kudlule ibhetri.I-voltage elungileyo ibuyisa ibhetri kwaye ibuyise umva ukuphendula kwamachiza phakathi kwe-electros elungileyo nengalunganga.I-elektrode eyamkelayo i-elektrode kwaye i-electrode engalunganga iwakhuphela.Impendulo yekhemikhali igcina amandla ombane njengamandla amandla emichiza, ukubuyisela ibhetri kwilizwe layo loqobo.I-IYO ihamba nge-electrolyte ukuba igcine ibhalansi.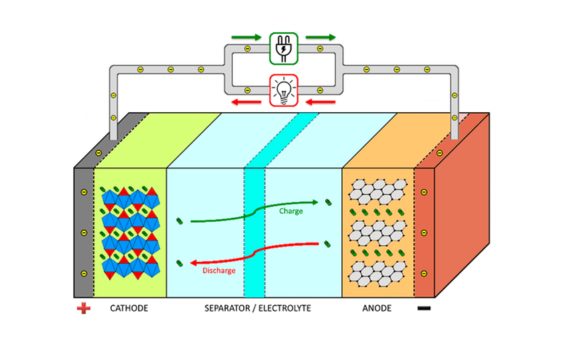
- I-voltage yebhetri, amandla, kunye noxinano lwamandla.
-
I-Voltage:
I-voltage ngumlinganiso wamandla okuphuma kombane webhetri.Ihlala ibonakaliswa kwiVolts.I-voltages eqhelekileyo yeseti yile ilandelayo:
•Ibhetri ye-lithium-ion (i-li-ion): Ngokubanzi 3.6 I-volts ukuya kwi-3.7 volts.Yintoni eyona nto ikhethekileyo kukuba i-Willpo4 (i-Lithium ye-Inyibilium phosphate) Ibhetri yi-3.2 volts.(i-voltage enye)
•Ibhetri ye-Nickel-Cadmium Cadmium (i-NICD): 1.2 Voltts (i-voltage yeseli).
•NI-Ckel-Metal Hydride (Nimh): 1.2 Voltts (i-voltage yeselula).
•Ibhetri ye-Acid-I-Acid-Acid): 2 Volts ukuya kwi-2.2 Volts (i-volts enye yeseli).Iibhetri ze-Acid-Acid ziqhele ukusetyenziswa kwimoto eqala, iinkqubo zokugcina amandla kunye namanye amasimi.
•Ibhetri ye-zinc-alkaline (i-winc-carbon): 1.5 Voltts (i-voltage yeseli).Olu hlobo lwebhetri luhlala lufumaneka kwiibhetri ezisebenzisa iAlkaline enye ezinjengeebhetri kunye neebhetri.
Oku kungasentla ngamavoti beebhetri ezahlukeneyo, kwaye sinokwandisa i-voltage ngokudibanisa uthotho.Imizekelo yile ilandelayo:
•Iibhetri ezintathu ze-3.7v Lithium-ion ixhunyiwe kuphuculo lokufumana ipakethi ye-11.1v Lithium-ion ye-ion ye-ion
•Iibhetri ezintathu ze-2V-acid-uqhagamshelo lwe-Acid yokufumana i-6V yebhetri ye-Acid-I-ACID;
• Iibhetri ezine ze-3.2v lithium ye-phosphatele prophatele ixhunyiwe kuphuculo lokufumana i-12.8V Lithium ye-Inyibium ye-phosphate yebhetri
Umthamo:
Xa uthetha ngomthamo webhetri, ihlala ibonakaliswa usebenzisa iyunithi ye-Ampre-iiyure (Ah) okanye iiyure ze-milliampere (Mah).Umthamo webhetri sisixa sentlawulo yokuba ibhetri ingayigcina kwaye inokuqondwa njengemveliso yangoku nexesha lokuba ibhetri inokuhambisa.Nantsi imizekelo eminye yemizekelo kunye nendlela abachazwa ngayo:
•I-2000 I-MAH ibhetri: Oku kuthetha ukuba ibhetri inomthamo wama-2000 Mah.Ukuba isixhobo sitsala umyinge ophakathi we-milliamps (Ma) ngeyure, emva koko ibhetri ingabonelela ngamandla ngamandla iiyure ezili-10 (i-2000mah / 200 ye-200 = iiyure ezili-10).
•Ibhetri ye-5h: oku kuthetha ukuba ibhetri inomthamo weeyure ezi-5 ze-AMP.Ukuba ifowuni ithatha umndilili we-1 AMP (a) ngeyure, emva koko le bhetri inokusebenza ngeyure ezi-5 (i-5ah / 1A = iiyure ezi-5).
Iipakethi zebhetri zinokuqhagamshelwa ngokuhambelana nezakhono zokunyuka komthamo, umzekelo:
•Iibhetri ezi-2 zeLi-ion ze-12v-100ah zinokuqhagamshelwa ngokuhambelana nokufumana ipakethi ye-12v-200a.
•Iibhetri ezi-2 ze-Fibrepo4 ze-3.2v-10ah zinokuqhagamshelwa ngokuhambelana nepakethi ye-Birpo4 ye-3.2v-20a.
I-1000 yam tshaja: Le yishaja enokuthi ihlawulise ibhetri ngereyithi ye-1000 yeemilimops (MI) ngeyure.Ukuba unebhetri ye-2000mAh, inyanzela ngale tshaja kuya kuthatha iiyure ezi-2 (i-2000mah / 1000ma = iiyure ezi-2) ukuze ihlawule ngokupheleleyo.
Ukuziqhelanisa, ixesha lokusetyenziswa kwethiyori ixesha lokusetyenziswa kwebhetri linokuphambuka ngenxa yebhetri kunye nokukrazula kunye nezinye izinto.
Ukuxinana kwamandla:
Ukuxinana kwamandla ngumgangatho wokusebenza kakuhle kwamandla agcinwe ebhetri.Ibonisa inani lamandla elinokugcinwa kwi-inter yeyunithi nganye okanye ubunzima beyunithi yebhetri.Iiyunithi eziqhelekileyo zokuxinana kwamandla ziyi-Watt-iyure nganye kwikhilogrem (i-wh / kg) okanye iyure yelitha nganye yelitha nganye (wh / l).
•Ibhetri ye-lithium-ion: I-Lithium-i-lithium-ion inoxinano lwamandla aphezulu, iqhele ukusukela kwi-150 ukuya kwi-250 wh / kg.
•Ibhetri ye-NIMH: UNimhbe iibhetri ze-nimh zine-xiness ephantsi ethelekiswa neLithium-i-ion-ion.Ngokwesiqhelo baqala ukusuka kwi-60 ukuya kwi-120 wh / kg.
•Ibhetri ye-ACID: Ibhetri ye-Acid-I-Acid-I-Acid-I-Acid-I-Acid-I-Acid-Acid inee-innsty eziphantsi zamandla xa kuthelekiswa neebhetri ze-lithium-ion.Baqala ukusuka kwi-30 ukuya kwi-50 wh / kg.
•Ibhetri ye-zinc-carbon: Iibhetri ze-zincBaqala ukusuka kwi-25 ukuya kwi-40 wh / kg.
- Iingcebiso zokugcina ibhetri
-
Ibhetri efanelekileyo ibalulekile ukugcina impilo yebhetri kwaye yandisa ubomi bayo.Nazi ezinye zeengcebiso zokugcina iibhetri:
•Iqondo lokushisa: Iibhetri zevenkile kwindawo epholileyo, eyomileyo eneqondo eliphakathi kwe-15 ° C kunye ne-25 ° C (59 ° F kwaye i-77 ° F).Amaqondo obushushu aphezulu anokukhawulezisa ireyithi yokuzikhupha kwaye anciphise ubomi beshelgi yebhendi.Kulumkele ukuveza iibhetri kubushushu obukhulu okanye ukubanda.
•Kuphephe ukufuma, umswakama unokuyonakalisa ibhetri, ekhokelela ekucocekeni okanye ukuvuza.Gcina iibhetri kude neendawo ezinomswakama, ezinjengamangaphantsi okanye amagumbi okuhlamba.Qinisekisa ukuba indawo yokugcina yomile kwaye ingena kakuhle.
•Inqanaba elihlawulelwayo: Ngaphambi kokugcina iibhetri ixesha elide, kungcono ukuqinisekisa ukuba bahlawuliswa ngokuyinxenye.Uninzi lwabavelisi bacebisa inqanaba elihlawulelwayo macala angama-40% ukuya kwi-60% yokugcina ixesha elide.Olu luhlu lunceda ukukhusela ngaphezulu-ukukhulula okanye ukwenzaka ngaphezulu kwemeko.
•Uhlobo lwebhetri: I-chemistries eyahlukileyo yebhetri ineemfuno ezithile zokugcina.Nazi ezinye izikhokelo zeentlobo eziqhelekileyo:
a. Iibhetri zeAlkaline: Iibhetri ze-alkaline zinobomi obude obude kwaye zinokugcinwa iminyaka eliqela.Azikhuseli kwaye akufuneki ziveze amaqondo obushushu agqithisileyo.
b. Ibhetri yeLithium-ion: I-Li-ion ibhetri idla ngokufuthi inamandla e-Engoma.Ukuba uceba ukubagcina ixesha elongeziweyo, ujolise kwinqanaba lentlawulo phakathi kwama-40% nama-60%.Kulumkele ukugcina ibhetri ye-ion kwityala elipheleleyo okanye ukhutshiwe ngokupheleleyo.
c. Iibhetri ze-Acid-Acid: Ezi zisetyenziswa rhoqo kwiinkqubo zemoto kunye neenkqubo ze-backup.Kwindawo yokuhlala yexesha elide, gcina iibhetri ze-acid-acid zihlawuliswa ngokupheleleyo.Jonga rhoqo amanqanaba e-electrolyte kwaye uphakamise amanzi anemidaka ukuba uyafuneka.
d. Ibhetri esekwe kwi-Nickel (i-nimh kunye ne-nind): I-Nigh kunye neebhetri ze-nidd kufuneka zigcinwe kwintlawulo ethile (malunga ne-40%).Ukuba ukhunjulwe ngokupheleleyo ngaphambi kokugcina, banokukhulisa uxinzelelo lwe-voltage, kunciphisa amandla abo.
•SUkugcinwa kokugcinwa: Iibhetri kwiindlela zokuthintela ukunxibelelana phakathi kwe-terminals yazo.Ukuba i-terminals elungileyo nengalunganga ichukumisana okanye inxibelelana nezinto ezenziwayo, kunokubangela ukukhululwa kunye nomonakalo onokubakho.
•Ukupakishwa kwasekuqaleni: Ukupakishwa kwasekuqaleni kwenzelwe ukukhusela iibhetri ezivela ekrwada, uthuli kunye nezinye izinto ezingcolisayo.
•Ukuhlolwa rhoqo: Vavanya iibhetri ezigciniweyo kuzo naziphi na iimpawu zokuvuza, ukususwa, okanye ukonakala.Ukuba uyayibona nayiphi na imiba, ubambe ngononophelo kwaye ulahle ngokufanelekileyo.
- Impembelelo yokusingqongileyo.
-
Ukurisayikilisha Ibhetri: Iibhetri zineekhemikhali ezahlukeneyo kunye nezinyithi ezinokuba yingozi kwindalo ukuba ingekho ngokufanelekileyo.Iibhetri zokuphinda zisebenzise kwakhona zinceda ukubuyisela izinto ezixabisekileyo ezinje ngeLithium, eCobabalt, kunye ne-nickel, kwaye kuthintele ukukhutshwa kwezinto eziyityhefu.Uluntu oluninzi lunemigangatho yokuphinda isebenze kwibhetri okanye iindawo zokulahla.Jonga kwabasemagunyeni okanye amaziko okurisimu ukuze ufumane ezinye iindlela zokulahla ezifanelekileyo kwindawo yakho.
Izinto ezinobungoziEzinye iibhetri, ezinje ngeebhetri ezikhokelayo ezisetyenziswa kwizithuthi, zinezinto ezinobungozi zifana ne-Cozardous Faide kunye ne-sulfuric acid.Ukulahlwa okungafanelekanga kwezi zibhetele kunokungcolisa umhlaba kunye nemithombo yamanzi, ibeka umngcipheko kwimpilo yabantu kunye nokusingqongileyo.Njengoko abantu beqonda ngakumbi ukhuseleko lwendalo, ngakumbi abantu abaninzi besebenzisa i-Lithium-i-lithium-i-ion-ion yendalo, ngakumbi iibhetri zobomi.
Ukusetyenziswa kwamandlaImveliso yebhetri ifuna amandla, kwaye impembelelo yokusingqongileyo iyahluka ngokuxhomekeke kuhlobo lwebhetri.Umzekelo, ukuveliswa kweebhetri ze-lithium-ion esetyenziswa kwizixhobo ezininzi ze-elektroniki kunye nezithuthi zombane kubandakanya ukukhutshwa kunye nokulungiswa kwezimbiwa.Sebenzisa izixhobo ezifanelekileyo amandla kunye nokusetyenziswa kwebhetri kunokunceda ukunciphisa ukusetyenziswa kwamandla ngokubanzi.
Imibhalo yenyawo yekhabhoni: Unyawo lwe-carbon oluhambelana nemveliso yebhetri kunye nokulahlwa inokuba negalelo kwimveliso yegesi yeGreenhouse kunye notshintsho lwemozulu.Ukwanda kwemithombo yamandla ahlaziyiweyo yokuphucula ibhetri kunye nokuphinda kusetyenziswe kwakhona kunokunceda ukunciphisa ifuthe lendalo.