Umhlahlandlela wokugcina kumabhethri
Umhlahlandlela olandelayo ufundise kakhulu, ngakho-ke sicela uthole lokho ofuna ukukufunda etafuleni lokuqukethwe ngokuya ngezinga lakho lolwazi lwebhethri.Vele uma ungumqali, sicela uqale ekuqaleni.
Ithiphu yokufunda kwangaphambili: Chofoza kanye ebhokisini lombhalo wesihloko futhi umbhalo onemininingwane uzokwanda;Chofoza futhi futhi umbhalo onemininingwane uzofihlwa.
Ukuqalisa
- Ukubaluleka nokusakazeka kwezicelo zamabhethri.
-
Amabhethri abaluleke kakhulu emphakathini wanamuhla futhi asetshenziswa ezinhlobonhlobo zezicelo (ngokuthuthukiswa kobuchwepheshe, amadivayisi amaningi aguqulwa abe amandla ebhethri).Bahlinzeka ngezixazululo zamandla okuphakelayo, ezingavuselelwa futhi eziphuthumayo abashayela ukuthuthukiswa kwezobuchwepheshe, ukusetshenziswa kwamandla okuzinzile kanye nenqubekela phambili ebangeni elihlukahlukene lezimboni.

1. Amadivayisi we-Electronic aphathekayo: Njengezingcingo ezifana, amaphilisi, ama-laptops namakhamera wedijithali.
2. Ukuthutha: Izimoto zikagesi kanye ne-hybrid zisebenzisa amabhethri njengethuluzi lokugcina lamandla.Ngesidingo esandayo samandla avuselelekayo kanye nezindlela zokuhamba ezinobungane zemvelo, amabhethri adlala indima ebalulekile ekushayeleni ukuthuthukiswa kwezokuthutha okuqhubekayo.
3. Isitoreji samandla esivuselelekayo: Amabhethri asetshenziswa kabanzi ukugcina imithombo yamandla evuselelwe njengamandla elanga nangomoya.Ngokugcina amandla kagesi kumabhethri, anganikeza ukunikezwa kukagesi okuqinile lapho amalanga elanga noma umoya engatholakali.
4. Amandla aphuthumayo: Amabhethri adlala indima ebalulekile njengomthombo wamandla we-back-up ezimweni eziphuthumayo.Isibonelo, amadivaysi afana nezingcingo ezingenazintambo, amasheya kanye namalambu aphuthumayo adinga amabhethri anikeze amandla athembekile.
5. Imishini yezokwelapha: Amadivaysi amaningi ezokwelashwa, anjenge-pacemaker kanye nama-ventialioners okufakelwa, asebenzise amabhethri njengomthombo wamandla.Ukuqina nokwethenjwa kwamabhethri kubalulekile ekusebenzeni kwalawa madivayisi abucayi.
6. Izicelo zezempi: Amabhethri asetshenziswa ezinhlobonhlobo zezicelo zezempi, ezifana nemishini yokuxhumana yezempi, amasistimu wokuhambisa kanye nama-drones.Amabhethri anganikeza ngokuhlinzekwa kwamandla ezizimele futhi athuthukise amakhono okulwa enkundleni yempi.
7. -Kwezimboni: Amabhethri asetshenziswa embonini yezinhlelo zebhethri, ama-emusman amoncu atholakalayo kanye nezinzwa ezingenantambo.Banikeza ukunikezwa kwamandla okuthembekile futhi baqinisekise ukuqhubeka nokuphepha kokukhiqizwa kwezimboni.
- Ukubuka konke kwezimiso eziyisisekelo nezinqubo ezisebenzayo zamabhethri.
-
Umlingo wamabhethri ulele emandleni abo okuguqula amandla amakhemikhali abe amandla kagesi.Ibhethri linama-electrodes amabili (amahle futhi abi) kanye ne-electrolyte.I-electrolyte isebenza njengomqhubi wama-ion, okwenza ukusabela kwamakhemikhali phakathi kwama-electrodes.
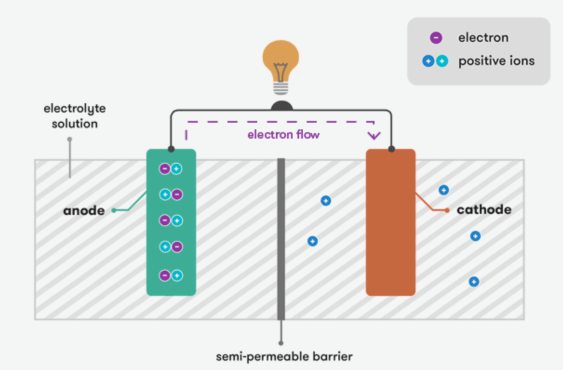
Isimiso esiyisisekelo sebhethri sisuselwa ekuphendukeni kwe-electrochemical.Lapho kwenzeka ukusabela kwamakhemikhali, kwakha ukugeleza kwama-elektroni.Esimweni esiqinisekisiwe, ibhethri ligcina amakhemikhali phakathi kwama-electrodes amahle nengelungile, futhi ukusabela kwamakhemikhali kubuyela emuva.Lapho ibhethri lixhumeke kwisekethe sangaphandle, ukusabela kwamakhemikhali kuqala, okwenza amakhemikhali e-terminal aqonde ku-oxidize kanye namakhemikhali e-terminal engemihle ukuze anciphise.Ngenxa yalokhu, ama-elektroni aphuma aqhamuka esigungwini esingesihle kwi-terminal evumayo, ekhiqiza i-Electric yamanje.Le nqubo iyaqhubeka kuze kube yilapho amakhemikhali eqediwe.
Izinhlobo ezahlukahlukene zamabhethri zisebenzisa ukusabela okuhlukile kwamakhemikhali ukukhiqiza ugesi.Isibonelo, uhlobo oluvame kakhulu lwebhethri le-lithium-ion: I-electrode yayo eyakhayo yenziwa nge-lithium compound (njenge-cobalt oxide noma i-lithium iron phosphate) kanye ne-electrode yalo engemihle yenziwa nge-carbon ebonakalayo (njenge-graphite).Esimweni esishadisiwe, ama-lithium ion afakwa ku-electrode avela kwizinto ezingezinhle.Ngesikhathi sokukhululwa, ama-lithium ion ashunyekwe kabusha kusuka ku-electrode engemihle futhi abuyele ku-elektrodi enhle, akhulula ama-elektroni.
- Inani lokuba nomhlahlandlela wokugcina kumabhethri wabafundi.
-
Umhlahlandlela wokugcina ubalulekile kumfundi ngezizathu eziningana:
1. Ukuhlinzeka ngemininingwane enembile: I-Intanethi igcwele izingcezwana zolwazi nemibono engqubuzanayo.Umhlahlandlela wokugcina uhlinzeka ngolwazi oluphelele nolunembile ngokuhlanganisa kanye nokuhlanganisa imithombo ethembekile ukusiza abafundi ukufinyelela ngokushesha ulwazi abaludingayo futhi bagweme imininingwane edukisayo noma engalungile.
2. Yonga isikhathi nomzamo: Ukucinga i-Intanethi ngezihloko ezithile kuvame ukudinga isikhathi esiningi sokukhipha futhi uqinisekise ukuthembeka kolwazi.Umhlahlandlela wokugcina usindisa isikhathi nomzamo ngokuhlanganisa imininingwane efanelekile ukuze abafundi bathole lonke ulwazi abaludingayo endaweni eyodwa.
3. Ukuxazulula Ukungqubuzana Nokudideka: I-Intanethi ivame ukuletha izimpendulo ezihlukile zombuzo noma ukuphikisana phakathi kwemininingwane.Umhlahlandlela wokugcina usiza abafundi ukubalekela ukudideka kanye nokuxakeka ngokuhlanganisa ukubukwa okuhlukile nemithombo egunyaziwe ukunikeza izimpendulo ezinokwethenjelwa kakhulu.
4. Hlinzeka ngokuholwa nezeluleko: Umhlahlandlela wokugcina awunikezi kuphela amaqiniso nolwazi, kepha futhi unganikeza isiqondiso esisebenzayo nezeluleko.
- Izinhlobo ezahlukahlukene zamabhethri: izimiso, izici, kanye nezicelo.
-
Nazi ezinye zezinhlobo ezi-5 ezivame kakhulu zamabhethri, kufaka phakathi izimiso zazo, izici kanye nezicelo.Uma ufuna imininingwane ebanzi kakhulu ngezinhlobo zebhethri, ungakwazi futhi ukweqa lesi sigaba bese uqonde ku- "Izinhlobo eziningi zebhethri kanye nezinhlelo zokusebenza" ngezansi.
Amabhethri e-lead-acid
•Isimiso: Amabhethri e-Holive-acid asebenzisa ukusabela kwamakhemikhali phakathi kokuhola nokuhola i-dioxide ukukhiqiza amandla kagesi.
•Izici: Izindleko eziphansi, eziphezulu zokuqala kwamanje kanye namandla, kepha ezinkulu futhi zisindayo.
•Izicelo: Amabhethri we-Stative Starter, ups (ukunikezwa kwamandla okungafinyeleleki), njll.
Amabhethri e-Li-ion (Lithium-ion)
•Isimiso: Amabhethri e-Lithium-Ion asebenzisa ukufuduka kwama-lithium ion phakathi kwama-electrodes amahle ne-negative okugcina nokukhipha amandla kagesi.
•Izici: Ubuningi bamandla aphezulu, isisindo esilula nempilo ende yokujikeleza.Ukushaja okuphezulu nokukhipha amandla.
•Izicelo: Amadivayisi eselula (e.g. Izingcingo eziphathwayo, amakhompyutha wethebhulethi), amadivaysi aphathekayo kagesi nezimoto zikagesi.
Amabhethri we-nicd (nickel-cadmium)
•Isimiso: Amabhethri we-NICD akhiqiza amandla kagesi ngokusabela kwamakhemikhali phakathi kwe-nickel ne-cadmium hydroxide.
•Izici: Ukukhishwa kwamandla aphezulu nempilo ende, kepha aqukethe i-cadmium eyingozi eyingozi, enomthelela othile emvelweni.
•Izicelo: Amakhamera edijithali, amathuluzi aphathekayo kanye nama-drones, njll.
Nimh (nickel-metal) amabhethri e-hydridi
• Isimiso: Amabhethri eNimh asebenzisa ukusabela kwamakhemikhali phakathi kwe-nickel ne-hydrogen ukugcina nokukhipha amandla kagesi.
•Izici: Ubuningi bamandla aphezulu, impilo ende, akukho ukungcoliswa kanye nokusebenza okungcono okuphezulu okuphezulu.
•Izicelo: Izimoto ezi-Hybrid, amasistimu wokugcina amandla, njll.
I-Lipo (Lithium Polymer) ibhethri

•Isimiso: I-Lithium Polymer Battery ifana ne-Lithium Ion Battery, kepha isebenzisa i-electrolyte eqinile ye-polymer esikhundleni se-electrolyte ye-liquid.
•Izici: Ubuningi obuphezulu bamandla, isisindo esilula, ukuphepha okungcono kanye nesilinganiso sokuzikhulula esiphansi.Ilungele amadivayisi amancane.
•Izicelo: Ama-laptops, amawashi ahlakaniphile namadivayisi wezokwelapha aphathekayo njll.
- Ulwazi lwe-Physics lwamabhethri
- Voltage (V):
I-Voltage imele umehluko ongaba khona kagesi phakathi kwamaphoyinti amabili esisekelweni.Kukalwa ngama-volts (v).Amandla kagesi ngaphesheya kwebhethri ngokuvamile aboniswa njenge-_batt.
Ukushaja (q):
Ukushaja kubhekisele enanini likagesi eligcinwe ebhethri.Kukalwa ngama-coulombs (c) noma amahora apholile (ah).Ubudlelwano phakathi kwecala nomthamo bunikezwa yi: Ukushaja (q) = umthamo (c) × valentage (v)
Amandla (C):
Amandla amelela inani lokushaja ibhethri elingasigcina.Ngokuvamile kulinganiswa kuma-ampere-amahora (ah) noma amahora ama-milliampere-amahora (mah).Ubudlelwano phakathi kwekhono, ukushaja, kanye namandla anikezwa ngu: Amandla (e) = umthamo (c) × valentage (v)
Amandla (e):
Amandla amandla okwenza umsebenzi noma amandla ohlelo lokudala izinguquko.Ngokwesimo samabhethri, amandla avame ukukalwa ngamahora watt-amahora (wh) noma ama-joules (j).Ubudlelwano phakathi kwamandla, umthamo, kanye necala kunikezwa ngu: I-Energy (e) = ukushaja (q) × valentage (v)
Amandla (P):
Amandla amele izinga lapho umsebenzi owenziwe noma amandla adluliselwe kuwo.Kukalwa kuma-watts (w).Amandla esifundeni abalwa kusetshenziswa ifomula: Amandla (P) = Voltage (v) × okwamanje (i)
Uchungechunge lwechungechunge:
1. Lapho amabhethri exhunywe ochungechungeni, i-voltage ephelele kuyo yonke isekethe yisamba se-voltages yebhethri ngalinye.Okwamanje kuhlala kufana.
Inani le-Voltage (V_Total) = V1 + V2 + v3 + ...
2. Lapho amabhethri exhunywe ochungechungeni, inani eliphelele liyisamba sebhethri ngalinye.Lokhu kungenxa yokuthi amanje ahlala enjalo, kepha inani eliphelele le-voltage landa.
Inani eliphelele (C_Total) = C1 + C2 + C3 + ...
Ukuxhumana okuhambisanayo:
1. Lapho amabhethri axhunyiwe ngokufana, inani eliphelele lihlala lifana nelo lebhethri ngalinye, ngenkathi inani lamanje likhona inani lemali le-currints ligeleza ngebhethri ngalinye.
Ingqikithi yamanje (I_Total) = I1 + I2 + I2 + ...
2. Lapho amabhethri axhunyiwe ngokufana, inani eliphelele lilingana nomthamo webhethri elilodwa.Lokhu kungenxa yokuthi i-voltage ihlala ifana, kepha inani eliphelele lamanje.
Inani eliphelele (C_Total) = C1 = C2 = C3 = ...
- Imibandela yebhethri ejwayelekile.
-
1. Umthamo webhethri: Inani lamandla kagesi ebhethri angawagcina, avame ukuvezwa kuma-amp-amahora (ah) noma ama-milli-amps (mah).
2. Amandla kagesi: Umehluko ongaba khona noma umehluko we-voltage webhethri, ovezwe ku-VOLTS V. Imelela inani lamandla kagesi ibhethri elingasigcina.
3. Iseli yebhethri: Iseli ngalinye ebhethri, equkethe i-electrode enhle, i-electrode engemihle ne-electrolyte.
4. Iphakethe lebhethri: Konke okubandakanya amaseli ebhethri ambalwa ahlanganisiwe.Imvamisa baxhumeke futhi balawulwa ngezixhumi, amabhodi wesifunda nezinye izinto.
5. Uchungechunge lwechungechunge: Amangqamuzana ebhethri amaningi axhunyiwe ngokulandelana, nge-terminal enhle exhunywe kwi-terminal engemihle, ukukhulisa i-voltage ephelele.Lapho uxhumeke ochungechungeni, ama-voltages weseli ahleliwe.
6. Ukuxhumana okuhambisanayo: Ixhuma amaseli amaningi webhethri ngokulandelana, nge-terminal enhle exhunywe kwi-terminal engemihle, ukukhulisa amandla aphelele futhi umthamo.Lapho uxhumeke ngokufana, amakhono amaseli ebhethri angezwa ndawonye.
7. Ukushaja: Ukudla amandla kagesi ebhethri kusuka emthonjeni wangaphandle ukuze ubuyisele amandla wamakhemikhali agcinwe ebhethri.
8. Dida: Ukukhishwa kwamandla kagesi kusuka ebhethri elizosetshenziswa ekunikezeni imishini kagesi noma amasekethe.
9. CISHE CYCLE: Kubhekisa ekushaja ngokuphelele kanye nenqubo yokukhipha.
10. Ukukhokhisa ukusebenza kahle: Isilinganiso phakathi kwamandla kagesi amunca ibhethri futhi amandla kagesi agcinwe empeleni ngesikhathi senqubo yokushaja.
11. Ukuzikhulula: Izinga lapho ibhethri lilahlekelwa amandla ngokwalo lapho lingasebenzi.
12. Impilo yebhethri: Isikhathi sokuphila sebhethri, esivame ukukalwa ngokwesibalo semijikelezo yokushaja noma isikhathi sokusetshenziswa.
13. Impilo yebhethri: Inani lesikhathi ibhethri elingaqhubeka nokunikeza amandla ngemuva kwenkokhiso eyodwa.
14. Ukushaja okusheshayo: Ubuchwepheshe bokushaja oletha amandla ebhethri ngokushesha ukunciphisa isikhathi sokushaja.
15. Uhlelo Lokuphathwa Kwebhethri (BMS): Uhlelo lukagesi oluqapha futhi lulawule isimo sebhethri, ukushaja kanye nokukhipha inqubo futhi kuvikela ibhethri ezimweni ezingezinhle njengokukhulisa ngokweqile kanye nokuxineka ngokweqile.
16. Impilo ye-Battery Cycle: Inombolo yemijikelezo yebhethri ingaqedela, imvamisa ilinganiswa ngokushaja kanye nokukhipha ukulahleka kwamandla okufana nama-80% umthamo wokuqala.
17. Inani eliphakeme lokushaja: Izinga eliphakeme lokushaja elingamukelwa ngokuphepha ngebhethri, elivezwe njengesilinganiso sendawo yokukhokhisa.
18. Isilinganiso sokukhipha esiphezulu: Isilinganiso esiphezulu samanje lapho ibhethri lingakhishwa ngokuphephile, livezwe njengesilinganiso samandla akhona.
19. Umjikelezo wokuvikela ibhethri: Idivaysi yokuphepha esetshenziselwa ukuqapha isimo sebhethri futhi inqamule umjikelezo webhethri uma kwenzeka ukwanda, ukweqisa, ukweqisa, ukweqisa, ukubhebhethekiswa kwebhethri.
20. I-Battery Polarity: Umehluko kanye nokukhonjwa phakathi kwama-terminals amahle nengalungile webhethri, evame ukukhonjiswa ngezimpawu + kanye - noma ukumaka.
21. Ukuvuselelwa kwebhethri: Inqubo yokulahla amabhethri asetshenzisiwe ukuze alulame futhi alahle izinto eziyingozi eziqukethwe kuzo futhi asebenzise kabusha izinto ezisetshenziswa kabusha.
22. Ukukhishwa okujulile: Isimo lapho ibhethri likhishwe khona ezingeni eliphansi kakhulu noma liphethwe ngokuphelele.Ukukhishwa okujulile akuvamisile ukunconywa kaningi ukugwema imiphumela emibi empilweni yebhethri.
23. Ukukhishwa okusheshayo: Indlela yokukhipha ekhipha amandla ebhethri endaweni ephakeme yamanje isikhashana esifushane.
24. Ukwehluleka kwebhethri: Isimo lapho ibhethri sehluleka ukunikeza amandla anele noma ukugcina ukusebenza okujwayelekile, okungadalwa ngenxa yezizathu ezahlukahlukene njengokuguga noma ukulimala.
25. I-Thermal Runaway : Kubhekiswa ekukhuphukeni okusheshayo nokungalawuleki kokushisa kwebhethri ngaphansi kwezimo ezingejwayelekile, njengokukhulisa, ukweqisa ngokweqile, ukugcwala ngokweqile, okungadala ukuthi ibhethri liqhume noma libambe umlilo.
26. Ama-elektrode ebhethri: Ama-elekthrothi amahle futhi alungile ebhethri, okuyizinto ezisemqoka zokugcina nokukhipha imali kagesi.
27. Isiteshi sokushintshana kwebhethri: Isikhungo noma insizakalo yokubuyiselwa okusheshayo kwamabhethri ezimotweni zikagesi ukuhlinzeka uhla olude.
28. Ukusabela kwe-electrochemical: Ukusabela kwamakhemikhali okwenzeka ebhethri ukuguqula amandla amakhemikhali abe amandla kagesi ngenqubo ye-redox.
29. I-electrolyte: Uketshezi olusebenzayo noma oluqinile olusetshenziselwa ukuthutha ama-ion phakathi kwama-electrodes amahle nengemuhle webhethri ukwenza lula ukusabela kwe-electrochemical.
30. Ijatshi: Idivaysi yokudlulisela amandla kagesi ebhethri ukuze ubuyisele amandla akhe amakhemikhali agcinwe.
31. Ukulinganisa kwebhethri: Inqubo lapho izindleko noma ukukhishwa kweseli ngalinye epakethe lebhethri kuyalungiswa ukuze kuqinisekiswe ukuthi icala lilinganiselwe phakathi kwamaseli ngamunye.
32. Ibhethri langaphandle: Iyunithi yebhethri elikhiphekayo elingaxhunywa kudivayisi ye-elekthronikhi ukuze inikeze amandla.
33. Inkomba yokushaja kwebhethri: Inkomba noma ukubonisa okukhombisa isimo sokushaja noma izinga lebhethri.
34. Umphumela wememori yebhethri: Isimo lapho umthamo webhethri uncipha kancane kancane njengoba amacala okukhokhiswayo futhi acule amaqhubu ayaphindwa, njengoba ibhethri likhumbula imali encane kanye nokukhipha amabanga amancane.
35. Umlinga: Kubhekiswa ekumelaneni kwangaphakathi kwebhethri, okuthinta ukusebenza kahle kwamandla kwalo kanye nokusebenza.
36. Ukuvikeke kakhulu: Umsebenzi noma idivaysi ebheka futhi ilawule izinga lokushisa lebhethri ukuvikela umonakalo omkhulu uma izinga lokushisa liphezulu kakhulu.
37. Ukuvikelwa okuphansi kweVoltage: Indlela yokuvikela esisika ngokuzenzakalelayo umjikelezo ukuvikela ukuphuma ngokweqile lapho i-battery voltage yehla ngaphansi komkhawulo ophephile.
38. Ukuvikelwa ngokweqile: Indlela yokuvikela esusa ngokuzenzakalelayo umjikelezo ukuvikela ukweqiwa lapho ibhethri ishaja ifinyelela umkhawulo wokuphepha.
39. Ukugcinwa kwebhethri: Inqubo yokugcina ibhethri esimweni esingasetshenziswanga isikhathi eside, imvamisa edinga izindlela ezifanele zokunciphisa ukuzimela nokuvikela ibhethri.
40. Uhlelo Lokuphathwa Kwebhethri (BMS): Uhlelo lwe-elekthronikhi lokuqapha, ukulawula nokuvikela isimo kanye nokusebenza kwephakethe lebhethri, okubandakanya ukuphathwa kwamanje, amavota, okushisa kanye namanye amapharamitha.
41. Inkomba Yezinga lebhethri: Idivaysi noma umsebenzi okhombisa izinga lokushaja okusele ebhethri, elivame ukuvezwa njengephesenti noma ngezigaba eziningana.
42. Isikhathi sokushaja: Isikhathi esidingekayo ukuletha ibhethri kusuka kwikhokhiso eliphansi ngecala eliphelele, elithonywa amandla weshaja kanye nomthamo webhethri.
43. Ukushisa okuhlangene: Ubudlelwano phakathi kokusebenza kwebhethri kanye nezinguquko ekushiseni, okungathinta umthamo, ukumelana kwangaphakathi kanye nezimpawu zokukhishwa kwebhethri.
44. Iwaranti yebhethri: Iwaranti Yomkhiqizi Kokusebenza Nekhwalithi yebhethri isikhathi esithile, evame ukuvezwa ezinyangeni noma eminyakeni.
45. Isiteshi Sokushaja: Imishini noma indawo esetshenziselwa ukuhlinzeka izimoto zikagesi noma enye imishini yebhethri yokushaja.
46. Umhloli webhethri: Idivaysi noma ithuluzi elisetshenziselwa ukukala i-voltage, umthamo, ukumelana kwangaphakathi namanye amapharamitha webhethri ukuhlola impilo nokusebenza kwawo.
47. Ukulinganisa okusebenzayo: Indlela yokuphatha ibhethri elingana necala le-Battery Pack ngokulawula amacala namazinga okuphuma phakathi kwamaseli ngamunye.
48. Ukulinganisa nje: Indlela yokuphatha ibhethri lapho inkokhiso ephaketheni lebhethri ilinganiselwe ngokuxhuma okuxhumayo noma ukushwabana okukhokhiswayo, imvamisa kungaphansi kokulinganisa okusebenzayo.
49. Ukupakishwa kwebhethri : Ukupakishwa kwangaphandle kwebhethri, esetshenziselwa ukuvikela iseli, kunikeze ukwesekwa kwesakhiwo nokuvimbela amasekethe amafushane.
50. Ubuningi Bamandla Abaphezulu: Inani eliphakeme lamandla kagesi ebhethri angawagcina ngevolumu ngayinye noma isisindo, ekhombisa ukusebenza kahle kwebhethri.
51. Izinga lokuzikhulula eliphansi: Izinga lapho ibhethri lilahlekelwa amandla kagesi ngokwalo lihamba kancane futhi ligcina isimo esiphakeme sokukhokhisa lapho ligcinwa noma lingasetshenziswa isikhathi eside.
52. I-Battery Polarization: Kubhekiswa ekushintsheni kwezinto ezingaphezulu kwama-electrodes ngesikhathi sokushaja nokukhipha ngenxa yokusabela kwamakhemikhali kuma-electrodes.
53. Ukuvuza kwe-electrolyte yebhethri: Isimo lapho i-electrolyte ebhethri ivuza endaweni yangaphandle, ezoholela ekuwohlokeni kokusebenza kwebhethri noma ezinye izinkinga zokuphepha.
54. Uhlelo lokupholisa ibhethri: Uhlelo olusetshenziselwa ukulawula izinga lokushisa lebhethri, kungaba ngokuhlanza ukushisa, fan noma ukupholisa uketshezi ukugcina ibhethri ngaphakathi kobubanzi bokushisa obufanele bokushisa.
55. Uhlelo lokushisa ibhethri: Uhlelo olusetshenziselwa ukuhlinzeka ngebhethri ezindaweni zokushisa eziphansi zokuqinisekisa ukusebenza kahle kwebhethri emazingeni okushisa aphansi.
56. Ibhethri eliphakeme lokucima okuphezulu: Ibhethri elikwazi ukuletha amandla kagesi endaweni yamanje yezicelo ezinezidingo zamandla aphezulu njengamathuluzi kagesi nezimoto zikagesi.
57. Ibhethri lesibili: Ibhethri elingakhiwa kabusha, ngokungafani nebhethri elawulwayo elingalungiswa.
58. Ukuqapha ibhethri: Idivaysi noma uhlelo lokuqapha isimo, i-voltage, izinga lokushisa kanye namanye amapharamitha webhethri ngesikhathi sangempela ukuhlinzeka ngolwazi futhi avikele ibhethri.
- Ukwakhiwa kwebhethri: Ama-electrodes, i-electrolyte, kanye nokwehlukanisa.
-
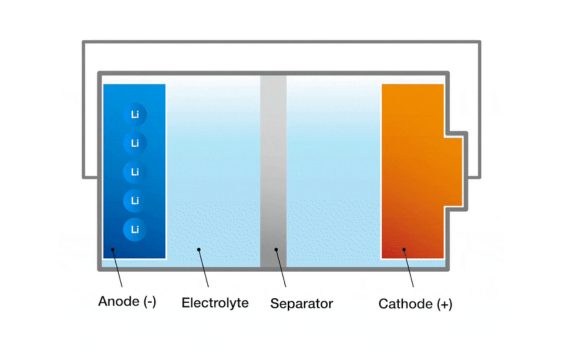
1. I-electrodes: Ama-electrodes ebhethri ahlukaniswe nge-electrode enhle futhi engemihle.I-electrode enhle kulapho ukusabela kwe-oxidation kwenzeka ebhethri kanye ne-electrode engemihle kulapho ukusabela kokuncipha kwenzeka ebhethri.Ama-electrodes amahle futhi alungile akhiwa ngezinto zokwenziwa, imvamisa izinsimbi, kusetshenziswa amakhabhoni noma amakhompiyutha.Umehluko mayelana nokuba khona phakathi kwama-electrodes amahle futhi amabi akhiqiza amavoti weseli lebhethri.
2. I-electrolyte: I-electrolyte iphakathi nendawo phakathi kwama-electrodes avumela ama-ion ukuthi adlule phakathi kwama-electrodes futhi agcine ibhalansi yecala.I-electrolyte ingaba ngoketshezi, ifomu eliqinile noma lejeli, kuya ngohlobo lweseli.Esitokisini se-liquid, i-electrolyte imvamisa i-ionic compound incibilika kusixazululo.
3. I-diaphragm: I-diaphragm iyisithiyo esingokomzimba phakathi kwama-elektrode amahle nengelungile, avikele ukugeleza kwe-elektroni eqondile kepha okuvumela ama-uons ukuthi adlule.Umsebenzi we-diaphragm ukuvikela ukujikeleza okufushane kwama-electrodes amahle futhi amabi ngenkathi uvumela ama-uons ukuthi ahambe ngokukhululeka nge-electrolyte futhi alondoloze ibhalansi yecala leseli.I-diaphragm imvamisa yenziwa ngendwangu ye-polymeric noma impahla ye-ceramic.
Lezi zingxenye zisebenza ngokubambisana ukwakha ukwakheka kweseli lebhethri.
- Izinqubo zokushaja nezokukhipha kumabhethri: Ukusabela kwamakhemikhali nokugeleza kwamanje.
-
1. Inqubo yokuphuma: Lapho ibhethri likhishwe, amandla amakhemikhali aguqulwa abe amandla kagesi.Ngesikhathi sokucasha, ukusabela koxidation kwenzeka endaweni eyakhayo kanye nokusabela kokuncipha esigungwini esingesihle.Ukuphendula kwamakhemikhali kukhiqiza ama-elektroni nama-ion.I-electrode enhle ikhipha ama-elektroni, ageleza ngomjikelezo wangaphandle ukuze akhiqize ugesi wamanje.I-electrode engemihle ithola ama-elektroni, ahlangana nama-ions ukwakha amakhompiyutha.Ngasikhathi sinye, u-Ion uhamba nge-electrolyte, egcina ibhalansi yebhethri.
2.Inqubo yokushaja: Ngesikhathi sokushaja kwebhethri, amandla kagesi aguqulwa abe amandla wamakhemikhali ukuze agcine amandla.Ngesikhathi senqubo yokushaja, umthombo wamandla wangaphandle usebenza nge-Forward Vertage, okubangela okwamanje ukudlula ebhethri.I-Voltage enhle ihlehlisa ibhethri futhi ibuyele ukusabela kwamakhemikhali phakathi kwama-electrodes amahle nengelungile.I-electrode enhle yamukela ama-elektroni futhi i-electrode engemihle isikhulule.Ukusabela kwamakhemikhali kugcina amandla kagesi njengamandla angaba khona amakhemikhali, ukubuyisela ibhethri esimweni salo sokuqala.I-Ions dlula nge-electrolyte ukuze ulondoloze ibhalansi yenkokhiso.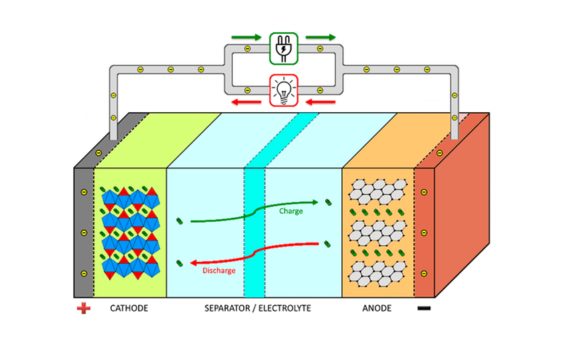
- Amandla webhethri, umthamo, kanye nobuningi bamandla.
-
I-Voltage:
I-Voltage iyisilinganiso samandla okukhipha kukagesi kwebhethri.Imvamisa kuvezwa ngama-volts.Ama-voltages ajwayelekile webhethri anjengokulandelayo:
•Ibhethri le-Lithium-Ion (Li-ion): Ngokuvamile 3.6 Volts kuya kuma-3.7 volts.Yini ekhethekile kakhulu ukuthi ibhethri le-LifePo4 (I-Lithium Iron Phosphate) lingama-3.2 volts.(I-Stoneli Voltage)
•I-Nickel-Cadmium Battery (NICD): 1.2 Volts (Voltage OLNE-Cell).
•NiI-ICLEL-Metal Hydride (NIMH): 1.2 Volts (Voltage Sholi voltage).
•I-Live-Acid Battery (Holy-Acid): 2 Volts kuya ku-2.2 volts (i-voltage eyodwa yeseli).Amabhethri e-lead-acid asetshenziswa kakhulu ekuqaliseni kwezimoto, amasistimu wokugcina amandla kanye nezinye izinkambu.
•Ibhethri le-Zinc-alkaline (i-zinc-carbon): 1.5 volts (volt-cell voltage).Lolu hlobo lwebhethri luvame ukutholakala kumabhethri e-alkaline asetshenziswayo asetshenziswayo afana namabhethri e-AA nama-AAA.
Lokhu okungenhla kukhona ama-voltage ahlukahlukene amabhethri ahlukahlukene, futhi singakhuphula amandla kagesi ngokuwaxhuma ngochungechunge.Izibonelo zinjengokulandelayo:
•Amabhethri amathathu we-3.7V we-lithium-ion axhunyiwe ku-Series ukuthola i-11.1V Lithium-Ion Battery Pack (okungukuthi, lokho esivame ukubiza ngebhethri yebhethri le-12V Lithium-Ion);
•Amabhethri amathathu we-2V Holy-acid axhunyiwe ku-Series ukuthola ipakethe lebhethri le-6V le-Acid;
• Amabhethri amane we-Iron we-Iron Phosphate axhunyiwe ku-Series ukuthola i-12.8V I-Iron Iron Phosphate Pherter Pack (okungukuthi, lokho esivame ukubiza i-12V Iron Iron Phosphate Battery Pack)
Amandla:
Lapho ukhuluma ngomthamo webhethri, kuvame ukuvezwa kusetshenziswa iyunithi yama-ampere-amahora (ah) noma amahora (ama-milliamppere-amahora.Umthamo webhethri inani lokushaja ukuthi ibhethri elingakwazi ukugcina futhi lingaqondakala futhi njengomkhiqizo wesikhathi nesikhathi lapho ibhethri lingaletha khona.Nazi ezinye izibalo zezibonelo nendlela achazwa ngayo:
•Ibhethri le-2000 MAH: Lokhu kusho ukuthi ibhethri linomthamo ka-2000 mah.Uma idivaysi idonsela i-milliamps yamanje yama-milliamps angama-200 ngehora, khona-ke leli bhethri linganikezela ngamandla emahoreni ayi-10 (2000mah / 200ma = amahora ayi-10).
•Ibhethri le-5Ah: Lokhu kusho ukuthi ibhethri linomthamo wama-5 amp-amahora.Uma idivaysi idla okuphakathi kwamanje kwe-1 amp (a) ngehora, khona-ke leli bhethri lingase linamandla wethiyori amahora ama-5 (5h / 1a = amahora ama-5).
Amaphakethe webhethri angaxhunywa ngokufana ukunikeza amandla andayo, ngokwesibonelo:
•Amabhethri ayi-2 we-Li-Ion we-12V-100AH angaxhunywa ngokufana ukuze athole iphakethe lebhethri le-li-ion le-12V-200ah.
•Amabhethri ayi-2 we-3.2v-10h angaxhunyaniswa ngokufana ukuze athole iphakethe lebhethri le-Lifepo4 le-3.2v-20h.
Ishaja yebhethri ye-1000mah: Le ishaja engakhokhisa ibhethri ngesilinganiso se-1000 milliamps (MA) ngehora.Uma unebhethri le-2000mah, lishaja ngalejajani kuzothatha amahora ama-2 (2000mah / 1000m / 1000m / 1000mA = amahora ama-2) ukuze ukhokhise ngokuphelele.
Ekusebenzeni, isikhathi esibalwe sebhethri esibalwe ngokweqiniso singashintsha ngenxa yokugqoka kwebhethri nokudabuka nezinye izinto.
Ubuningi bamandla:
Ubuningi bamandla isilinganiso sokusebenza kahle kwamandla agcinwe ebhethri.Kubonisa inani lamandla angagcinwa ngevolumu ngalinye noma isisindo seyunithi yebhethri.Amayunithi ajwayelekile wobuningi bamandla kuyihora le-watt ngekhilogremu ngalinye (wh / kg) noma ihora le-watt ilitha (wh / l).
•Ibhethri le-Lithium-Ion: Amabhethri e-Lithium-Ion anobunzima obuphakeme wamandla, ngokujwayelekile asukela ku-150 kuya ku-250 wh / kg.
•Ibhethri le-NIMH: Amabhethri eNimh anobunzima obuphansi wamandla uma kuqhathaniswa namabhethri e-lithium-ion.Ngokuvamile zisukela kuma-60 kuye kwayi-120 wh / kg.
•I-Live-Acid Battery: Amabhethri e-Holid-acid anobunzima obuphansi bamandla uma kuqhathaniswa namabhethri e-lithium-ion.Ngokuvamile zisukela kuma-30 kuye kwangama-50 wh / kg.
•Ibhethri le-zinc-carbon: amabhethri e-zinc-carbon anokungezelela kwamandla okuphansi kuqhathaniswa namabhethri e-lithium-ion.Ngokuvamile basukela ku-25 kuye ku-40 wh / kg.
- Izincomo zokugcina ibhethri
-
Ukulondolozwa kwebhethri okufanele kubalulekile ukuze ulondoloze impilo yebhethri futhi wandise isikhathi sawo sokuphila.Nazi ezinye izincomo zokugcina amabhethri:
•Ukushisa: gcina amabhethri endaweni epholile, eyomile enamazinga okushisa phakathi kuka-15 ° C no-25 ° C (59 ° F no-77 ° F).Amazinga okushisa aphezulu angasheshisa izinga lokuzikhulula futhi anciphise impilo yebhethri.Gwema ukudalula amabhethri ekushiseni okwedlulele noma kubanda.
•Gwema umswakama: Umswakama ungalimaza amabhethri, okuholela ekugcekeni noma ukuvuza.Gcina amabhethri kude nezindawo ezinomswakama, njengokungaphansi noma ezindlini zokugezela.Qinisekisa ukuthi indawo yokugcina yeyomile futhi ingena kahle.
•Izinga lokushaja: Ngaphambi kokugcina amabhethri isikhathi eside, kungcono ukuqinisekisa ukuthi akhokhiswa ngokwengxenye.Iningi labakhiqizi lincoma izinga lokushaja cishe cishe ama-40% kuya ku-60% ukuze kugcinwe isikhathi eside.Leli banga lisiza ukuvimbela ukuphuma ngokweqile noma izimo ezikhulayo ngesikhathi sokugcina.
•Uhlobo lwebhethri: I-Battery Cermissies ehlukile inezidingo ezithile zesitoreji.Nazi ezinye izinkombandlela zezinhlobo ezijwayelekile:
a. Amabhethri e-Alkaline: Amabhethri e-Alkaline anempilo ende eshalofini futhi angagcinwa iminyaka eminingana.Azikhokhiswa futhi akufanele zivezwe amazinga okushisa athe xaxa.
b. Amabhethri e-Lithium-Ion: Amabhethri e-Li-Ion ajwayele amandla kagesi aphathekayo.Uma uhlela ukuwagcina isikhathi eside, ahlose ukuthola izinga lokushaja phakathi kwama-40% no-60%.Gwema ukugcina amabhethri e-Li-Ion ngokugcwele noma akhishwe ngokuphelele.
c. Amabhethri e-lead-acid: Lezi zivame ukusetshenziswa ezimotweni nezinhlelo zamandla ezipele.Ukuze ugcine isikhathi eside, gcina amabhethri e-lead-acid akhokhiswe ngokugcwele.Hlola njalo amazinga we-electrolyte bese ubheka amanzi aphukile uma kudingeka.
d. Amabhethri asuselwa ku-nickel (NIMH ne-NICD): Amabhethri aseNim kanye nama-NICD kufanele agcinwe kwicala elithile (cishe ama-40%).Uma zidedelwa ngokuphelele ngaphambi kokugcina, zingakha ukudangala kwamandla kagesi, zinciphisa amandla azo.
•UshikukaziIsitoreji se-EPARATE: izitolo zamabhethri ngendlela evimbela ukuxhumana phakathi kwama-terminals abo.Uma ama-terminals afanele futhi amabi athintana noma axhumane nezinto zokwakha, kungadala ukukhishwa kanye nomonakalo ongaba khona.
•Ukupakishwa koqobo: Ukupakishwa kwangempela kwenzelwe ukuvikela amabhethri kusuka kumswakama, uthuli nakwezinye izinto ezingcolile.
•Ukuhlolwa okujwayelekile: Ngezikhathi ezithile kuhlolwe amabhethri agciniwe nganoma yiziphi izimpawu zokuvuza, ukugqwala, noma ukulimala.Uma ubona noma yiziphi izingqinamba, zibaphathe ngokunakekela bese uzilahla kahle.
- Umthelela Wezemvelo.
-
Ukuvuselelwa kwebhethri: Amabhethri aqukethe amakhemikhali ahlukahlukene nezinsimbi ezingaba yingozi emvelweni uma zingalahlwa kahle.Amabhethri asebenzisa kabusha asiza ukubuyisa izinto ezibalulekile njenge-lithium, cobalt, kanye ne-nickel, futhi ivimbele ukukhishwa kwezinto ezinobuthi.Imiphakathi eminingi inezinhlelo zokuvuselelwa kabusha kwebhethri noma izindawo zokulahla.Bheka neziphathimandla zasendaweni noma izikhungo zokuphinda usebenzise kabusha ukuthola izinketho zokulahla kahle endaweni yangakini.
Izinto ezinobungozi: Amanye amabhethri, njengamabhethri e-lead-acid asetshenziswa ezimotweni, aqukethe izinto eziyingozi njengokuhola ne-sulfuric acid.Ukulahlwa okungafanele kwalawa mabhethri kungangcolisa imithombo yenhlabathi kanye namanzi, kubeka engcupheni impilo yabantu kanye nemvelo.Njengoba abantu beqala ukuqaphela ngokwengeziwe ukuvikela kwezemvelo, abantu abaningi basebenzisa amabhethri amaningi e-lithium-ion, ikakhulukazi amabhethri we-Lifepo4.
Ukusetshenziswa kwamandla: Ukukhiqizwa kwebhethri kudinga amandla, futhi umthelela wezemvelo uyahlukahluka ngokuya ngohlobo lwebhethri.Isibonelo, ukukhiqizwa kwamabhethri we-lithium-ion asetshenziswa kumadivayisi amaningi we-elekthronikhi nezimoto zikagesi kubandakanya ukukhishwa nokucutshungulwa kwamaminerali.Kusetshenziswa amadivaysi asebenza kahle kwamandla nokwenza kahle ukusetshenziswa kwebhethri kungasiza ukunciphisa ukusetshenziswa kwamandla okuphelele.
I-Carbon Footprint: I-footprint yekhabhoni ehambisana nokukhiqizwa kwebhethri nokulahlwa kwemali kungaba nomthelela ekuphumeni kwamagesi abamba ukushisa kanye nokushintsha kwesimo sezulu.Ukwanda ukwamukelwa kwemithombo yamandla evuselelekayo yokukhiqiza ibhethri nokuphinda usebenzise kabusha kungasiza ukunciphisa umthelela wezemvelo.