The Ultimate Guide To Batteries
The following guide is very informative, so please find what you want to learn from the table of contents depending on your level of battery knowledge. Of course if you are a beginner, then please start at the beginning.
Pre-reading tip: Click once on the title text box and the detailed text will expand; click again and the detailed text will be hidden.
Introduction
- The importance and widespread applications of batteries.
-
Batteries are very important in modern society and are used in a wide range of applications (with the development of technology, more and more devices are being converted to battery power). They provide portable, renewable and emergency power solutions that drive technological development, sustainable energy use and progress in a wide range of industries.

1. Portable electronic devices: Such as mobile phones, tablets, laptops and digital cameras.
2. Transportation: Electric and hybrid vehicles use batteries as the primary energy storage device. With the increased demand for renewable energy and environmentally friendly modes of transport, batteries are playing a key role in driving sustainable transport development.
3. Renewable energy storage: Batteries are widely used to store renewable energy sources such as solar and wind power. By storing electrical energy in batteries, they can provide a steady supply of electricity when solar or wind energy is not available.
4. Emergency power: Batteries play an important role as a back-up power source in emergency situations. For example, devices such as cordless phones, torches and emergency lights require batteries to provide reliable power.
5. Medical equipment: Many medical devices, such as pacemakers and artificial ventilators, use batteries as a power source. The stability and reliability of batteries is critical to the operation of these critical devices.
6. Military applications: Batteries are used in a wide range of military applications, such as for military communication equipment, navigation systems and drones. Batteries can provide an independent energy supply and enhance combat capabilities on the battlefield.
7. Industrial: Batteries are used in industry for battery systems, emergency power supplies and wireless sensors. They provide a reliable power supply and ensure the continuity and safety of industrial production.
- An overview of the fundamental principles and working mechanisms of batteries.
-
The magic of batteries lies in their ability to convert chemical energy into electrical energy. A battery comprises two electrodes (positive and negative) and an electrolyte. The electrolyte acts as a conductor of ions, enabling a chemical reaction between the electrodes.
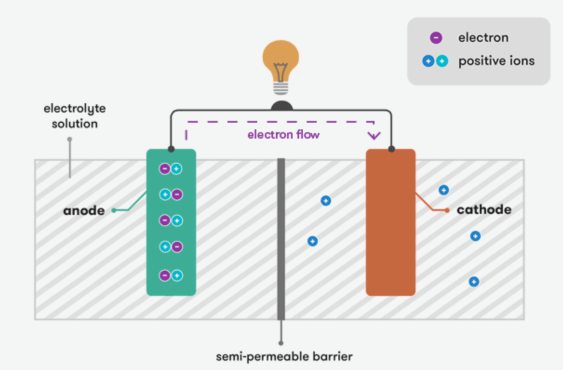
The fundamental principle of a battery is based on electrochemical reactions. When a chemical reaction occurs, it generates the flow of electrons. In the charged state, the battery stores chemicals between the positive and negative electrodes, and the chemical reaction is reversible. When the battery is connected to an external circuit, the chemical reaction begins, causing the chemical at the positive terminal to oxidize and the chemical at the negative terminal to reduce. As a result, electrons flow from the negative terminal to the positive terminal, producing an electric current. This process continues until the chemicals are depleted.
Different types of batteries employ distinct chemical reactions to generate electricity. For example, the most common type of lithium-ion battery: its positive electrode is made up of a lithium compound (such as cobalt oxide or lithium iron phosphate) and its negative electrode is made up of a carbon material (such as graphite). In the charged state, lithium ions are embedded from the positive electrode into the negative material. During discharge, the lithium ions are de-embedded from the negative electrode and return to the positive electrode, releasing electrons.
- The value of having an ultimate guide on batteries for the readers.
-
An ultimate guide is valuable to the reader for several reasons:
1. To provide accurate information: The Internet is full of information fragments and conflicting opinions. An Ultimate Guide provides comprehensive and accurate information by consolidating and collating reliable sources to help readers quickly access the knowledge they need and avoid misleading or incorrect information.
2. Save time and effort: Searching the Internet for specific topics often requires a great deal of time to sift through and verify the reliability of information. The Ultimate Guide saves time and effort by bringing together relevant information so that readers can find all the information they need in one place.
3. Resolving contradictions and confusion: The Internet often presents different answers to the same question or contradictions between information. The Ultimate Guide helps readers to escape confusion and bewilderment by synthesising different views and authoritative sources to give the most reliable answers.
4. Provide guidance and advice: The Ultimate Guide not only provides facts and information, but can also provide practical guidance and advice.
- Different types of batteries: principles, characteristics, and applications.
-
Here are some of the 5 most common types of batteries, including their principles, characteristics and applications. If you want the most comprehensive information on battery types, you can also skip this section and go straight to "Most Battery Types and Applications" below.
Lead-acid batteries
• Principle: Lead-acid batteries use a chemical reaction between lead and lead dioxide to produce electrical energy.
• Features: Low cost, high starting current and energy density, but large and heavy.
• Applications: automotive starter batteries, UPS (Uninterruptible Power Supply), etc.
Li-ion (Lithium-ion) batteries
• Principle: Lithium-ion batteries use the migration of lithium ions between positive and negative electrodes to store and release electrical energy.
• Features: High energy density, lighter weight and longer cycle life. High charging and discharging efficiency.
• Applications: Mobile devices (e.g. mobile phones, tablet computers), portable electronic devices and electric vehicles.
NiCd (Nickel-cadmium) batteries
• Principle: NiCd batteries produce electrical energy through a chemical reaction between nickel and cadmium hydroxide.
• Features: High power output and long life, but they contain the harmful heavy metal cadmium, which has a certain impact on the environment.
• Applications: digital cameras, portable tools and drones, etc.
NiMH (Nickel-metal) hydride batteries
• Principle: NiMH batteries use the chemical reaction between nickel and hydrogen to store and release electrical energy.
• Features: high energy density, long life, no pollution and better high temperature performance.
• Applications: Hybrid vehicles, energy storage systems, etc.
LiPo (Lithium polymer) battery

• Principle: The lithium polymer battery is similar to the lithium ion battery, but it uses a solid polymer electrolyte instead of a liquid electrolyte.
• Features: High energy density, lighter weight, better safety and lower self-discharge rate. Suitable for thin devices.
• Applications: Laptops, smart watches and portable medical devices etc.
- Physics Knowledge of Batteries
- Voltage (V):
Voltage represents the electric potential difference between two points in a circuit. It is measured in volts (V). The voltage across a battery is typically denoted as V_batt.
Charge (Q):
Charge refers to the amount of electric charge stored in a battery. It is measured in coulombs (C) or ampere-hours (Ah). The relationship between charge and capacity is given by: Charge (Q) = Capacity (C) × Voltage (V)
Capacity (C):
Capacity represents the amount of charge a battery can store. It is typically measured in ampere-hours (Ah) or milliampere-hours (mAh). The relationship between capacity, charge, and energy is given by: Energy (E) = Capacity (C) × Voltage (V)
Energy (E):
Energy is the capacity to do work or the potential for a system to cause changes. In the context of batteries, energy is often measured in watt-hours (Wh) or joules (J). The relationship between energy, capacity, and charge is given by: Energy (E) = Charge (Q) × Voltage (V)
Power (P):
Power represents the rate at which work is done or energy is transferred. It is measured in watts (W). The power in a circuit is calculated using the formula: Power (P) = Voltage (V) × Current (I)
Series Connection:
1. When batteries are connected in series, the total voltage across the circuit is the sum of the individual battery voltages. The current remains the same.
Total Voltage (V_total) = V1 + V2 + V3 + ...
2. When batteries are connected in series, the total capacity is the sum of the individual battery capacities. This is because the current remains the same, but the total voltage increases.
Total Capacity (C_total) = C1 + C2 + C3 + ...
Parallel Connection:
1. When batteries are connected in parallel, the total voltage remains the same as that of an individual battery, while the total current is the sum of the currents flowing through each battery.
Total Current (I_total) = I1 + I2 + I3 + ...
2. When batteries are connected in parallel, the total capacity is equal to the capacity of a single battery. This is because the voltage remains the same, but the total current increases.
Total Capacity (C_total) = C1 = C2 = C3 = ...
- Common battery terms and definitions.
-
1. Battery Capacity: The amount of electrical energy that a battery can store, usually expressed in amp-hours (Ah) or milli-amps (mAh).
2. Voltage: The potential difference or voltage difference of a battery, expressed in volts V. It represents the amount of electrical energy the battery can store.
3. Battery Cell: An individual cell in a battery, containing the positive electrode, negative electrode and electrolyte.
4. Battery Pack: A whole consisting of several battery cells combined. They are usually connected and managed through connectors, circuit boards and other components.
5. Series Connection: Multiple battery cells connected in sequence, with the positive terminal connected to the negative terminal, to increase the total voltage. When connected in series, the cell voltages are superimposed.
6. Parallel Connection: Connects multiple battery cells in sequence, with the positive terminal connected to the negative terminal, to increase the total current capability and capacity. When connected in parallel, the capacities of the battery cells are added together.
7. Charging: Feeding electrical energy into the battery from an external source to restore the chemical energy stored in the battery.
8. Discharging: The release of electrical energy from a battery for use in supplying electronic equipment or circuits.
9. Charge Cycle: Refers to a complete charging and discharging process.
10. Charge Efficiency: The ratio between the electrical energy absorbed by the battery and the electrical energy actually stored during the charging process.
11. Self-discharge: The rate at which a battery loses power on its own when not in use.
12. Battery Life: The life span of a battery, usually measured in terms of the number of charge cycles or usage time.
13. Battery Life: The amount of time a battery can continue to supply power after a single charge.
14. Fast Charging: A charging technology that delivers power to the battery faster to reduce charging time.
15. Battery Management System (BMS): An electrical system that monitors and controls the condition of the battery, the charging and discharging process and protects the battery from adverse conditions such as overcharge and overdischarge.
16. Battery Cycle Life: The number of charge cycles a battery can complete, usually measured by charging and discharging to a specific capacity loss such as 80% of the original capacity.
17. Maximum Charge Rate: The maximum charge rate that can be safely accepted by the battery, expressed as a ratio of the charge capacity.
18. Maximum Discharge Rate: The maximum current rate at which a battery can be safely discharged, expressed as a ratio of current capacity.
19. Battery Protection Circuit: A safety device used to monitor the condition of the battery and to disconnect the battery circuit in the event of overcharge, overdischarge, overcurrent, overtemperature, etc. to prevent damage or danger to the battery.
20. Battery Polarity: The distinction and identification between the positive and negative terminals of a battery, usually indicated by the symbols + and - or markings.
21. Battery Recycling: The process of disposing of used batteries in order to recover and dispose of the hazardous materials contained in them and to reuse recyclable materials.
22. Deep Discharge: A condition in which a battery is discharged to a very low level or completely depleted. Deep discharge is not usually recommended frequently to avoid negative effects on battery life.
23. Rapid Discharge: A discharge technique that releases the battery's energy at a high current for a short period of time.
24. Battery Failure: A condition where the battery is unable to provide sufficient power or maintain normal operation, which can be caused by various reasons such as ageing or damage.
25. Thermal Runaway: Refers to the rapid and uncontrollable rise in temperature of a battery under abnormal conditions, such as overcharge, overdischarge, overheating, etc., which may cause the battery to explode or catch fire.
26. Battery Electrodes: The positive and negative electrodes in a battery, which are the key components for storing and releasing electrical charge.
27. Battery Swapping Station: A facility or service for the rapid replacement of batteries in electric vehicles to provide longer range.
28. Electrochemical Reaction: The chemical reaction that takes place in a battery to convert chemical energy into electrical energy through a redox process.
29. Electrolyte: A conductive liquid or solid used to transport ions between the positive and negative electrodes of a battery to facilitate the electrochemical reaction.
30. Charger: A device for transferring electrical energy to a battery to restore its stored chemical energy.
31. Battery Balancing: A process by which the charge or discharge rate of each cell in a battery pack is adjusted to ensure that the charge is balanced between the individual cells.
32. External Battery: A removable battery unit that can be connected to an electronic device to supply power.
33. Battery Charging Indicator: An indicator or display that shows the state of charge or level of a battery.
34. Battery Memory Effect: A phenomenon whereby the capacity of a battery gradually decreases as the charge and discharge cycles are repeated, as the battery remembers the smaller charge and discharge ranges.
35. Impedance: Refers to the internal resistance of a battery, which affects its energy conversion efficiency and performance.
36. Temperature Protection: A function or device that monitors and controls the temperature of a battery to prevent overheating damage if the temperature becomes too high.
37. Low Voltage Protection: A protection mechanism that automatically cuts the circuit to prevent over-discharge when the battery voltage drops below a safe threshold.
38. Overcharge Protection: A protection mechanism that automatically cuts off the circuit to prevent overcharge when the battery charge reaches the safety threshold.
39. Battery Storage: The process of retaining a battery in an unused state for an extended period of time, often requiring appropriate measures to reduce self-discharge and protect the battery.
40. Battery Management System (BMS): An electronic system for monitoring, controlling and protecting the condition and performance of a battery pack, including the management of current, voltage, temperature and other parameters.
41. Battery Level Indicator: A device or function that indicates the level of charge remaining in a battery, usually expressed as a percentage or in several stages.
42. Charging Time: The time required to bring a battery from a low charge to a full charge, which is influenced by the power of the charger and the capacity of the battery.
43. Temperature Coefficient: The relationship between battery performance and changes in temperature, which can affect the capacity, internal resistance and charge/discharge characteristics of the battery.
44. Battery Warranty: A manufacturer's warranty on the performance and quality of a battery for a certain period of time, usually expressed in months or years.
45. Charging Station: An equipment or facility used to supply electric vehicles or other battery equipment for charging.
46. Battery Tester: A device or instrument used to measure the voltage, capacity, internal resistance and other parameters of a battery to assess its health and performance.
47. Active Balancing: A battery management technique that equalises the charge in a battery pack by controlling the charge and discharge rates between the individual cells.
48. Passive Balancing: A battery management technique in which the charge in a battery pack is balanced by connecting resistors or charge leakage, usually less efficiently than active balancing.
49. Battery Packaging: The external packaging of a battery, used to protect the cell, provide structural support and prevent short circuits.
50. High Energy Density: The maximum amount of electrical energy that a battery can store per unit volume or weight, indicating the energy storage efficiency of the battery.
51. Low Self-Discharge Rate: The rate at which a battery loses electrical energy on its own is very slow and maintains a high state of charge when stored or unused for a long period of time.
52. Battery Polarization: Refers to the change in material on the surface of the electrodes during charging and discharging due to chemical reactions on the electrodes.
53. Battery Electrolyte Leakage: A condition in which the electrolyte in a battery leaks into the external environment, which will result in degradation of battery performance or other safety problems.
54. Battery Cooling System: A system used to control the temperature of a battery, either through heat dissipation, fan or liquid cooling to keep the battery within the appropriate operating temperature range.
55. Battery Heating System: A system used to provide heat to the battery in low temperature environments to ensure proper operation of the battery at low temperatures.
56. High Discharge Rate Battery: A battery that is capable of delivering electrical energy at a high current for applications with high power requirements such as power tools and electric vehicles.
57. Secondary Battery: A battery that can be recharged, as opposed to a disposable battery that is not rechargeable.
58. Battery Monitor: A device or system for monitoring the status, voltage, temperature and other parameters of a battery in real time to provide information and protect the battery.
- Battery construction: electrodes, electrolyte, and separator.
-
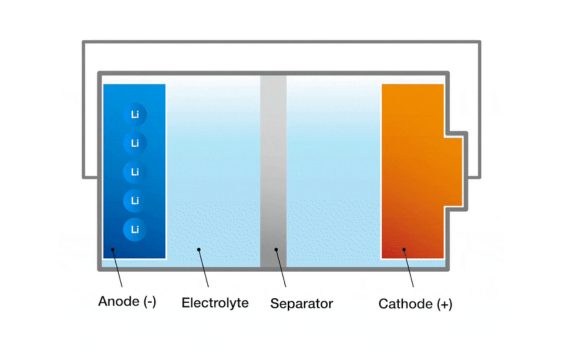
1. Electrodes: The electrodes in a battery are divided into a positive and a negative electrode. The positive electrode is where the oxidation reaction takes place in the battery and the negative electrode is where the reduction reaction takes place in the battery. The positive and negative electrodes are made up of conductive materials, usually metals, carbon or compounds are used. The difference in potential between the positive and negative electrodes produces the voltage of the battery cell.
2. Electrolyte: The electrolyte is the medium between the electrodes which allows ions to pass between the electrodes and maintains the charge balance. The electrolyte can be in liquid, solid or gel form, depending on the type of cell. In a liquid cell, the electrolyte is usually an ionic compound dissolved in solution.
3. Diaphragm: The diaphragm is a physical barrier between the positive and negative electrodes, preventing direct electron flow but allowing ions to pass through. The function of the diaphragm is to prevent short-circuiting of the positive and negative electrodes while allowing ions to move freely through the electrolyte and maintaining the charge balance of the cell. The diaphragm is usually made of a polymeric material or a ceramic material.
These components work together to form the structure of the battery cell.
- Charge and discharge processes in batteries: chemical reactions and current flow.
-
1. Discharge process: When a battery is discharged, chemical energy is converted into electrical energy. During discharge, an oxidation reaction takes place at the positive terminal and a reduction reaction at the negative terminal. The chemical reactions produce electrons and ions. The positive electrode releases electrons, which flow through an external circuit to produce an electric current. The negative electrode receives electrons, which combine with ions to form compounds. At the same time, ions move through the electrolyte, maintaining the charge balance of the battery.
2.Charging process: During the charging of a battery, electrical energy is converted into chemical energy in order to store energy. During the charging process, an external power source applies a forward voltage, causing a current to pass through the battery. The positive voltage reverses the battery and reverses the chemical reaction between the positive and negative electrodes. The positive electrode accepts electrons and the negative electrode releases them. The chemical reaction stores electrical energy as chemical potential energy, restoring the battery to its original state. Ions move through the electrolyte to maintain charge balance.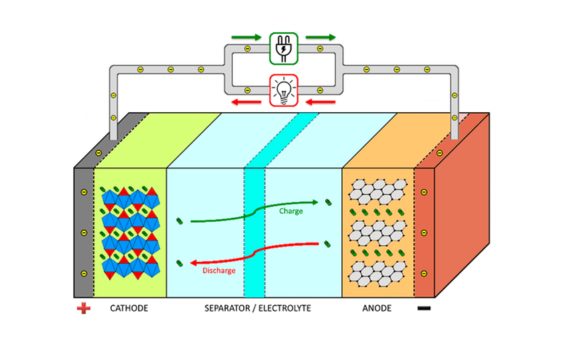
- Battery voltage, capacity, and energy density.
-
Voltage:
Voltage is a measure of the strength of a battery's electrical output. It is usually expressed in volts. Common battery cell voltages are as follows:
• Lithium-ion battery (Li-ion) : Generally 3.6 volts to 3.7 volts . What is more special is that the LiFePO4 (lithium iron phosphate) battery is 3.2 volts. (single cell voltage)
• Nickel-cadmium battery (NiCd): 1.2 volts (single-cell voltage).
• Nickel-metal hydride (NiMH): 1.2 volts (single-cell voltage).
• Lead-acid battery (Lead-Acid): 2 volts to 2.2 volts (single cell voltage). Lead-acid batteries are commonly used in automobile starting, energy storage systems and other fields.
• Zinc-Alkaline battery (Zinc-Carbon): 1.5 volts (single-cell voltage). This type of battery is commonly found in single-use alkaline batteries such as AA and AAA batteries.
The above are the voltages of various batteries, and we can also increase the voltage by connecting them in series. Examples are as follows:
• Three 3.7v lithium-ion batteries are connected in series to get an 11.1v lithium-ion battery pack (that is, what we often call a 12v lithium-ion battery pack);
• Three 2v lead-acid batteries are connected in series to get a 6v lead-acid battery pack;
• Four 3.2V lithium iron phosphate batteries are connected in series to get a 12.8v lithium iron phosphate battery pack (that is, what we often call a 12v lithium iron phosphate battery pack)
Capacity:
When talking about battery capacity, it is often expressed using the unit of ampere-hours (Ah) or milliampere-hours (mAh). Battery capacity is the amount of charge that a battery can store and can also be understood as the product of the current and time that the battery can deliver. Here are some example figures and the way they are described:
• 2000 mAh battery: This means that the battery has a capacity of 2000 mAh. If the device draws an average current of 200 milliamps (mA) per hour, then this battery can theoretically supply power for 10 hours (2000mAh / 200mA = 10 hours).
• 5Ah battery: This means that the battery has a capacity of 5 amp-hours. If the device consumes an average current of 1 amp (A) per hour, then this battery can theoretically power for 5 hours (5Ah / 1A = 5 hours).
Battery packs can be connected in parallel to give an increased capacity, for example:
• 2 Li-ion batteries of 12V-100Ah can be connected in parallel to get a Li-ion battery pack of 12V-200Ah.
• 2 LiFePO4 batteries of 3.2V-10Ah can be connected in parallel to get a LiFePO4 battery pack of 3.2V-20Ah.
1000mAh battery charger: This is a charger that can charge the battery at a rate of 1000 milliamps (mA) per hour. If you have a 2000mAh battery, charging it with this charger will theoretically take 2 hours (2000mAh / 1000mA = 2 hours) to fully charge it.
In practice, the theoretically calculated battery usage time may deviate due to battery wear and tear and other factors.
Energy density:
Energy density is a measure of the efficiency of the energy stored in a battery. It indicates the amount of energy that can be stored per unit volume or unit weight of the battery. Common units of energy density are watt-hour per kilogram (Wh/kg) or watt-hour per liter (Wh/L).
• Lithium-ion battery: Lithium-ion batteries have high energy density, typically ranging from 150 to 250 Wh/kg.
• NiMH battery: NiMH batteries have a lower energy density compared to lithium-ion batteries. They typically range from 60 to 120 Wh/kg.
• Lead-acid battery: Lead-acid batteries have relatively low energy density compared to lithium-ion batteries. They typically range from 30 to 50 Wh/kg.
• Zinc-carbon battery: Zinc-carbon batteries have lower energy density compared to lithium-ion batteries. They typically range from 25 to 40 Wh/kg.
- Battery storage recommendations
-
Proper battery storage is essential to maintain battery health and prolong its lifespan. Here are some recommendations for storing batteries:
• Temperature: Store batteries in a cool, dry place with a temperature between 15°C and 25°C (59°F and 77°F). High temperatures can accelerate the self-discharge rate and shorten the battery's shelf life. Avoid exposing batteries to extreme heat or cold.
• Avoid Humidity: Moisture can damage batteries, leading to corrosion or leakage. Keep batteries away from humid environments, such as basements or bathrooms. Ensure the storage area is dry and well-ventilated.
• Charge Level: Before storing batteries for an extended period, it's best to ensure they are partially charged. Most manufacturers recommend a charge level of around 40% to 60% for long-term storage. This range helps prevent over-discharge or overcharge conditions during storage.
• Battery Type: Different battery chemistries have specific storage requirements. Here are some guidelines for common types:
a. Alkaline Batteries: Alkaline batteries have a long shelf life and can be stored for several years. They are not rechargeable and should not be exposed to extreme temperatures.
b. Lithium-ion Batteries: Li-ion batteries commonly power portable electronics. If you plan to store them for an extended period, aim for a charge level between 40% and 60%. Avoid storing Li-ion batteries at full charge or completely discharged.
c. Lead-acid Batteries: These are commonly used in vehicles and backup power systems. For long-term storage, keep lead-acid batteries fully charged. Regularly check the electrolyte levels and top up with distilled water if needed.
d. Nickel-based Batteries (NiMH and NiCd): NiMH and NiCd batteries should be stored at a partial charge (around 40%). If they are fully discharged before storage, they may develop voltage depression, reducing their overall capacity.
• Separate Storage: Store batteries in a way that prevents contact between their terminals. If positive and negative terminals touch each other or come into contact with conductive materials, it can cause discharge and potential damage.
• Original Packaging: The original packaging is designed to protect the batteries from moisture, dust, and other contaminants.
• Regular Inspection: Periodically inspect stored batteries for any signs of leakage, corrosion, or damage. If you notice any issues, handle them with care and dispose of them properly.
- Environmental impact.
-
Battery recycling: Batteries contain various chemicals and metals that can be harmful to the environment if not properly disposed of. Recycling batteries helps recover valuable materials like lithium, cobalt, and nickel, and prevents the release of toxic substances. Many communities have battery recycling programs or drop-off locations. Check with local authorities or recycling centers to find the proper disposal options in your area.
Hazardous substances: Some batteries, such as lead-acid batteries used in vehicles, contain hazardous substances like lead and sulfuric acid. Improper disposal of these batteries can contaminate soil and water sources, posing a risk to human health and the environment. As people become more aware of environmental protection, more and more people are using more environmentally friendly lithium-ion batteries, especially LiFePO4 batteries.
Energy consumption: Battery production requires energy, and the environmental impact varies depending on the battery type. For example, the production of lithium-ion batteries used in many electronic devices and electric vehicles involves the extraction and processing of minerals. Using energy-efficient devices and optimizing battery usage can help reduce overall energy consumption.
Carbon footprint: The carbon footprint associated with battery production and disposal can contribute to greenhouse gas emissions and climate change. Increased adoption of renewable energy sources for battery manufacturing and recycling can help mitigate the environmental impact.